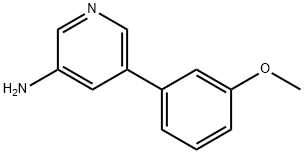
5-(3-Methoxyphenyl)pyridin-3-aMine synthesis
- Product Name:5-(3-Methoxyphenyl)pyridin-3-aMine
- CAS Number:1225523-08-9
- Molecular formula:C12H12N2O
- Molecular Weight:200.24
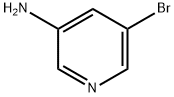
13535-01-8
381 suppliers
$3.00/1g
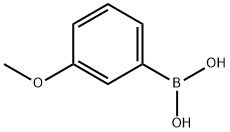
10365-98-7
405 suppliers
$9.00/10g

1225523-08-9
8 suppliers
inquiry
Yield:1225523-08-9 91%
Reaction Conditions:
with tetrakis(triphenylphosphine) palladium(0);potassium carbonate in methanol;water;toluene at 100; for 40 h;
Steps:
Synthesis of N-(5-(3-methoxyphenyl)pyridin-3-yl)cyclopropanecarboxamide 19
The mixture of (3-methoxyphenyl)boronic acid 22 (1.0 g, 6.6 mmol), 5-bromopyridin-3-amine 23 (1.0 g, 5.8 mmol), Pd(PPh3)4 (0.2 g, 0.17 mmol) and K2CO3 (1.5 g, 10.9 mmol) in toluene (12.0 mL), MeOH (5.0 mL) and water (6.0 mL) was sealed and heat at 100 oC for 40 hrs. The mixture was cooled to rt. The catalyst was filter off through a layer of Celite. The filtrate was diluted with ethyl acetate (50.0 mL), washed with water, brine, dried over Na2SO4 and concentrated under reduced pressure. The resulting residue was purified by CombiFlash (CH2Cl2/MeOH: 100/0 to 95/5 in 20 min) to afford 1.05 g (91%) of 5-(3-methoxyphenyl)pyridin-3-amine 24. 1H NMR (400 MHz, CDCl3): δ 8.21 (d, J = 2.1 Hz, 1H), 8.04 (d, J = 3.2 Hz, 1H), 7.32 (t, J = 8.1 Hz, 1H), 7.02-7.10 (m, 3H), 6.87-6.91 (m, 1H), 3.92 (bs, 2H), 3.81 (s, 3H). 5-(3-methoxyphenyl)pyridin-3-amine 24 (0.240 g, 1.20 mmol) was dissolved in CH2Cl2 (10.0 mL) and cooled at 0 oC. Triethylamine (0.17 mL, 1.20 mmol) was added, followed by the addition of cyclopropanecarbonyl chloride 13 (0.125 g, 1.20 mmol). The mixture was stirred at rt for 2 hrs. The solvent was removed under reduced pressure. The resulting residue was purified with chromatography (CH2Cl2/MeOH: 100/0 to 95/5 in 20 min) to afford 0.250 g (78%) of the desired product 19.
References:
Li, Guiying;Zhou, Hao;Jiang, Yu;Keim, Holger;Topiol, Sidney W.;Poda, Suresh B.;Ren, Yong;Chandrasena, Gamini;Doller, Darío [Bioorganic and Medicinal Chemistry Letters,2011,vol. 21,# 4,p. 1236 - 1242] Location in patent:supporting information; experimental part