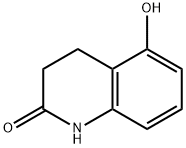
5-Hydroxy-3,4-dihydroquinolin-2(1H)-one synthesis
- Product Name:5-Hydroxy-3,4-dihydroquinolin-2(1H)-one
- CAS Number:30389-33-4
- Molecular formula:C9H9NO2
- Molecular Weight:163.17
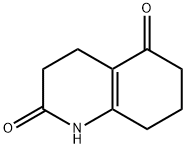
5057-12-5
66 suppliers
$84.00/1g

30389-33-4
203 suppliers
$15.00/250mg
Yield:30389-33-4 92.7%
Reaction Conditions:
with N-Bromosuccinimide in cyclohexane; for 3 h;Cooling with ice;Reflux;Solvent;
Steps:
5.c Step c: Preparation of 5-hydroxy-3,4-dihydro-2 (1H) -quinolone
To a 100 ml three-necked flask were added 3,4,7,8-tetrahydro-2,5 (1 H, 6Η) -quinoline dione (0.02 mol) and cyclohexane (25 ml) After stirring to dissolve in the ice bath for 10min, slowly add dropwise a solution of N-bromosuccinimide (0.03mol) in cyclohexane (5ml), and reflux for 3h at the end of the dropwise addition. After completion of the reaction by TLC, cool down to room temperature . Water was added (amount was 0.7 times the volume of the reactant), and the mixture was allowed to stand with stirring. The aqueous layer was extracted with ethyl acetate (1 × the volume of the aqueous layer 4 times), and the organic layers were combined. Anhydrous sulfuric acid Calcium was dried and filtered, the filtrate was evaporated to remove the solvent, and the residue was dried to give an off-white solid as a 5-hydroxy-Dihydro-2 (1H) -quinolone (Yield 92.7%), mp 233 ~ 234 °C, BP2013-HPLC method chromatography purity 94.3%.
References:
CN107337639,2017,A Location in patent:Paragraph 0120; 0127; 0139; 0150; 0161; 0171; 0172; 0183

21261-76-7
44 suppliers
$60.00/500mg

22246-18-0
616 suppliers
$5.00/5g

30389-33-4
203 suppliers
$15.00/250mg

5057-12-5
66 suppliers
$84.00/1g

31570-97-5
105 suppliers
$72.00/100mg

30389-33-4
203 suppliers
$15.00/250mg

30557-06-3
26 suppliers
inquiry

30389-33-4
203 suppliers
$15.00/250mg