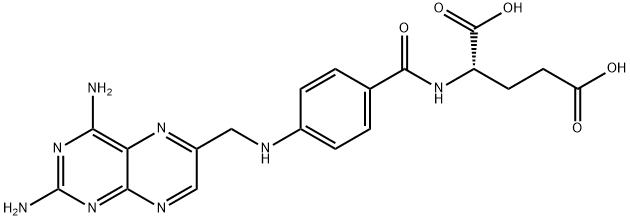
Aminopterin synthesis
- Product Name:Aminopterin
- CAS Number:54-62-6
- Molecular formula:C19H20N8O5
- Molecular Weight:440.41
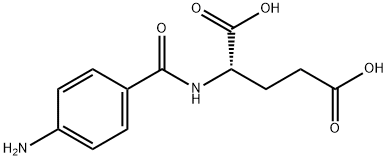
4271-30-1
358 suppliers
$6.00/1g

7732-18-5
458 suppliers
$12.69/100ml
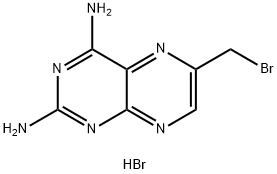
52853-40-4
158 suppliers
$21.00/250mg

54-62-6
204 suppliers
$25.00/10mg
Yield:54-62-6 68%
Reaction Conditions:
in ethanol
Steps:
3 N-[4-[[(2,4-Diamino-6-pteridinyl)methyl]amino]benzoyl]-L-glutamic Acid (3, Aminopterin) Hydrate (4:7).
EXAMPLE 3. N-[4-[[(2,4-Diamino-6-pteridinyl)methyl]amino]benzoyl]-L-glutamic Acid (3, Aminopterin) Hydrate (4:7). A mixture of I (168 mg, 0.500 mmole) and N-(4-aminobenzoyl)-L-glutamic acid (400 mg, 1.50 mmoles) in DMAC (2 ml) was stirred at 25° under N2 in a stoppered flask protected from light. Solution occurred after 2 hrs. After 18 hrs, the orange solution was mixed with H2 O (15 ml) with stirring to give a finely divided, yellow precipitate. The mixture was centrifuged, and the supernatant removed by decantation. The yellow solid was stirred with four 15-ml portions of H2 O, each of which was removed by decantation after centrifugation. The solid was then suspended in EtOH (15-20 ml), collected by filtration, washed with Et2 O, and dried in vacuo (25°, P2 O5) to give hydrated 3 in 68% yield (160 mg). Anal. Calcd for C19 H20 N8 O5.1.75H2 O: C, 48.36; H, 5.02; N, 23.74. Found: C, 48.72; H, 4.91; N, 23.36.
References:
The United States of America as represented by the Department of Health, Education and Welfare US4077957, 1978, A
59-30-3
1111 suppliers
$5.00/5g

54-62-6
204 suppliers
$25.00/10mg
![4-(N-[2,4-DIAMINO-6-PTERIDINYLMETHYL]-AMINO)BENZOIC ACID SODIUM SALT](/CAS/GIF/36093-85-3.gif)
36093-85-3
18 suppliers
inquiry

54-62-6
204 suppliers
$25.00/10mg

945-24-4
141 suppliers
$38.00/25mg

54-62-6
204 suppliers
$25.00/10mg
