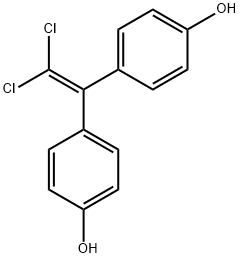
Bisphenol C synthesis
- Product Name:Bisphenol C
- CAS Number:14868-03-2
- Molecular formula:C14H10Cl2O2
- Molecular Weight:281.13
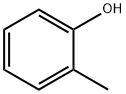
95-48-7
434 suppliers
$17.00/25g

67-64-1
6 suppliers
$17.30/10ml

14868-03-2
88 suppliers
$55.20/100 mg
Yield:14868-03-2 210 g
Reaction Conditions:
with hydrogenchloride;1-dodecylthiol in toluene at 30; for 10 h;Inert atmosphere;
Steps:
1-3; 1
(1) First step (1-1) Preparation of mixed solution In a separable flask equipped with a hydrogen chloride blowing tube, a thermometer, a jacket and an anchor-type stirring blade, 510 g (4.7 mol) of orthocresol, 104 g (1.8 mol) of acetone, 100 g of toluene and dodecanethiol under a nitrogen atmosphere. 10 g was added and the internal temperature was adjusted to 30 ° C. to prepare a mixed solution.(1-2) Reaction Hydrogen chloride gas was slowly bubbled into the mixed solution and then reacted for 10 hours to obtain a reaction solution.(2) Second step After adding 720 g of toluene and 500 g of desalinated water to the obtained reaction solution, the internal temperature was raised to 80 ° C. with stirring. After the internal temperature reached 80 ° C., the mixture was allowed to stand and separated into a first organic phase and a first aqueous phase to obtain a first organic phase.When the first aqueous phase was extracted and the acid concentration was measured, it was 2.1 mmol-NaOH / g.(3) First washing step 250 g of desalted water was added to 1400 g of the obtained first organic phase, and after the internal temperature reached 80 ° C., the mixture was allowed to stand to separate into a second organic phase and a second aqueous phase, and the second water was separated. By extracting the phase, a second organic phase was obtained.(4) Alkaline cleaning process To 1400 g of the obtained second organic phase, 300 g of a 10 mass% sodium hydrogen carbonate aqueous solution was added, and after the internal temperature reached 80 ° C. while mixing, the mixture was allowed to stand and the lower third aqueous phase (sodium hydrogen carbonate) was allowed to stand. It was confirmed that the pH of the aqueous phase) was 9 or more. Then, the third organic phase and the third aqueous phase of the upper layer were separated from each other, and the third aqueous phase was extracted to obtain a third organic phase.(5) Purification process The obtained third organic phase is cooled from 80 ° C. to 10 ° C., and after reaching 10 ° C., solid-liquid separation is performed using centrifugation (2500 rpm, 10 minutes) to obtain the first wet cake. Obtained. The obtained first wet cake was transferred to a beaker, 500 g of toluene was added thereto, and suspension washing was performed. The obtained slurry liquid was subjected to solid-liquid separation again using centrifugation (2,500 rpm, 10 minutes) to obtain 415 g of a second wet cake.A part of the second wet cake (300 g) and toluene (420 g) were placed in a full jacket type separable flask equipped with a thermometer and a stirrer, and the temperature was raised to 80 ° C. After confirming that the solution was uniform, a fourth organic phase was obtained. Further, 200 g of desalinated water was added to the obtained fourth organic phase and mixed for 30 minutes to remove the fourth aqueous phase of the lower phase to obtain a fifth organic phase. The pH of the removed fourth aqueous phase was 9. 200 g of desalted water was added to the obtained fifth organic phase and mixed for 30 minutes to remove the fifth aqueous phase of the lower phase to obtain a sixth organic phase. Further, 200 g of desalinated water was added to the obtained sixth organic phase and mixed for 30 minutes to remove the sixth aqueous phase of the lower phase to obtain a seventh organic phase. The electrical conductivity of the sixth aqueous phase (immediately preceding aqueous phase) was 1.9 μS / cm.The obtained seventh organic phase was cooled from 80 ° C. to 10 ° C. Then, filtration was carried out using a centrifuge (3000 rpm for 10 minutes) to obtain wet purified bisphenol C. Using an evaporator equipped with an oil bath, a light boiling point was distilled off at an oil bath temperature of 80 ° C. under reduced pressure to obtain 210 g of white bisphenol C.When the methanol-dissolved color of the obtained bisphenol C was measured, the number of Hazen colors was 5. Moreover, when the melt color difference of the obtained bisphenol C was measured, the number of Hazen colors was 25. Further, when the thermal color stability of the obtained bisphenol C was measured, the number of Hazen colors was 45. Moreover, when the thermal decomposition stability of the obtained bisphenol C was measured, the amount of isopropenyl cresol produced was 162 mass ppm.
References:
Mitsubishi Chemical Holdings Corp;Uchiyama, Kaoru;Nakajima, Yukie;Kishida, Shin JP2020/152650, 2020, A Location in patent:Paragraph 0123-0139
2971-36-0
20 suppliers
$115.00/500 mg

14868-03-2
88 suppliers
$55.20/100 mg
2132-70-9
20 suppliers
$110.00/50mg

14868-03-2
88 suppliers
$55.20/100 mg
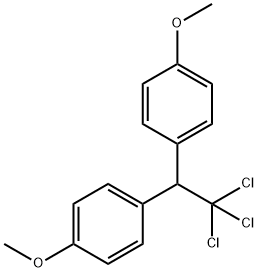
72-43-5
87 suppliers
$37.89/5g

14868-03-2
88 suppliers
$55.20/100 mg
![Phenol, 2-[2,2-dichloro-1-(4-hydroxyphenyl)ethenyl]-](/CAS/20210111/GIF/71032-16-1.gif)
71032-16-1
0 suppliers
inquiry

108-95-2
717 suppliers
$14.00/25g
![4-[2,2-Dichloro-1-(4-methoxyphenyl)ethenyl]phenol](/CAS/20180527/GIF/75938-34-0.gif)
75938-34-0
0 suppliers
inquiry

14868-03-2
88 suppliers
$55.20/100 mg