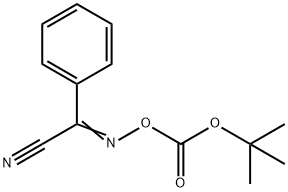
Boc-beta-alanine synthesis
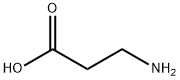
- Product Name:Boc-beta-alanine
- CAS Number:3303-84-2
- Molecular formula:C8H15NO4
- Molecular Weight:189.21
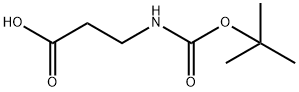
![ethyl 3-{[(tert-butoxy)carbonyl]amino}propanoate](/CAS/20180703/GIF/88574-53-2.gif)
88574-53-2
32 suppliers
$90.00/250 mg

3303-84-2
355 suppliers
$6.00/5g
Yield:3303-84-2 90%
Reaction Conditions:
with water;lithium hydroxide in ethanol at 20; for 5 h;
Steps:
Intermediate I-i 3B: 3 -((tert-butoxycarbonyl)amino)propanoic acid
Ethyl 3-((tert-butoxycarbonyl)amino)propanoate (1.4, 6.44 mmol) was dissolved in ethanol (10 mL) and a solution of lithium hydroxide (0.154 g, 6.44 mmol) in water (10 mL) was added. The reaction mixture was stirred at room temperature for 5 hrs. The reaction mixture was concentrated in vacuo to provide a cmde residue which wasdissolved in water and washed with DCM. The aqueous layer was neutralized with 6NHC1 and the solid obtained was filtered and dried under vacuum to afford 3-((tert-butoxycarbonyl)amino)propanoic acid (1.1 g, 90 %yield) as a white solid. LCMS m/z188.2 (M-H); ‘HNMR(400 MHz, MeOD) ö 3.29 (t,J= 11.20Hz, 2H), 2.47 (t,J= 13.60Hz, 2H), 1.43 (s, 9H).
References:
BRISTOL-MYERS SQUIBB COMPANY;DUNCIA, John V.;GARDNER, Daniel S.;HYNES, John;MACOR, John E.;MURUGESAN, Natessan;SANTELLA, Joseph B.;WU, Hong;KANTHETI, Durgarao;NAIR, Satheesh Kesavan;PAIDI, Venkatram Reddy;RATNA KUMAR, Sreekantha;SARKUNAM, Kandhasamy;SISTLA, Ramesh Kumar;POLIMERA, Subba Rao WO2016/210036, 2016, A1 Location in patent:Page/Page column 121

24424-99-5
816 suppliers
$13.50/25G
107-95-9
721 suppliers
$5.00/10g

3303-84-2
355 suppliers
$6.00/5g

58885-58-8
179 suppliers
$10.00/1g

3303-84-2
355 suppliers
$6.00/5g