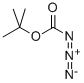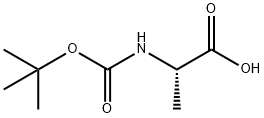
N-(tert-Butoxycarbonyl)-L-alanine synthesis
- Product Name:N-(tert-Butoxycarbonyl)-L-alanine
- CAS Number:15761-38-3
- Molecular formula:C8H15NO4
- Molecular Weight:189.21
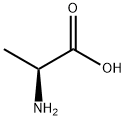
56-41-7
772 suppliers
$5.00/5g

24424-99-5
816 suppliers
$13.50/25G

15761-38-3
512 suppliers
$5.00/10g
Yield:15761-38-3 100%
Reaction Conditions:
with sodium hydroxide in tetrahydrofuran;water at 20; for 17 h;
Steps:
4.2 Step 2 (S)-2-((tert-butoxycarbonyl)amino)propanoic acid
To a suspension of (S)-2-aminopropanoic acid (10.00 g, 112.24 mmol) in H2O (56 mL) was added NaOH (6.73 g, 168.36 mmol) at 0° C., followed by adding 56 mL of THF and (Boc)2O (31.85 g, 145.91 mmol) via funnel. The resulted solution was stirred at rt for 17 hours, and extracted with petroleum ether (100 mL×2). The aqueous layer was acidified to pH=1 with 4 M HCl aqueous solution, then was extracted with EtOAc (100 mL×4). The combined organic phase was washed with 100 mL of saturated brine, dried over anhydrous Na2SO4, and concentrated in vacuo to give the title compound as colorless oil that was used for the next step without further purification (21.2 g, 100%). [0537] 1H NMR (600 MHz, CDCl3) δ (ppm): 9.95 (br. s, 1H), 5.19-5.18 (d, J=5.5 Hz, 1H), 4.32-4.31 (m, 1H), 1.41 (s, 9H), 1.41-1.39 (d, J=7.8 Hz, 3H).
References:
CALITOR SCIENCES, LLC;SUNSHINE LAKE PHARMA CO., LTD.;Xi, Ning;Wang, Liang;Wang, Tingjin US2015/87658, 2015, A1 Location in patent:Paragraph 0536; 0537

28875-17-4
140 suppliers
$6.00/5g

15761-38-3
512 suppliers
$5.00/10g
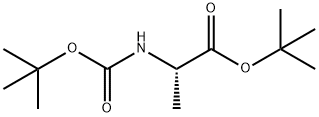
58177-77-8
29 suppliers
inquiry

15761-38-3
512 suppliers
$5.00/10g