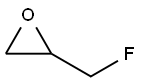
Epifluorohydrin synthesis
- Product Name:Epifluorohydrin
- CAS Number:503-09-3
- Molecular formula:C3H5FO
- Molecular Weight:76.07
106-89-8
730 suppliers
$9.00/10 g

503-09-3
75 suppliers
$40.00/1 g
Yield: 88%
Reaction Conditions:
with C6H11BFO3(1-)*K(1+) in N,N-dimethyl-formamide at 85; for 2 h;
Steps:
33
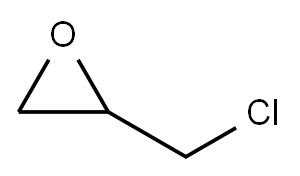
EXAMPLE 33 This example and the following two examples illustrate the use of the complexes of the invention for “Halex”-type chlorine→fluorine exchanges in which the complex is used in a stoichiometric amount so as to allow reactions which are impossible with the prior art owing to the sensitivity of the substrates to the elimination reactions.20 g of epichlorohydrin and 44 g of K+[C2H5C(CH2O)3BF]-, which is the adduct formed by the IB-2 compound with KF, are added to 150 ml of DMF and brought to 85° C. KCl is gradually formed and the reaction mixture is filtered after two hours of stirring. 14 g of epifluorohydrin (88% yield) are obtained by precipitation from 500 ml of water and then filtration.
References:
Armand, Michel;Tarascon, Jean-Marie;Recham, Nadir;Grugeon, Sylvie;Laruelle, Stephane;Devaraj, Shanmukaraj US2011/171112, 2011, A1 Location in patent:Page/Page column 23

818-92-8
19 suppliers
inquiry

15464-00-3
0 suppliers
inquiry

503-09-3
75 suppliers
$40.00/1 g

3031-75-2
3 suppliers
inquiry

75-07-0
411 suppliers
$14.00/5mL
67-63-0
1548 suppliers
$16.00/25ML

67-64-1
6 suppliers
$17.30/10ml
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
