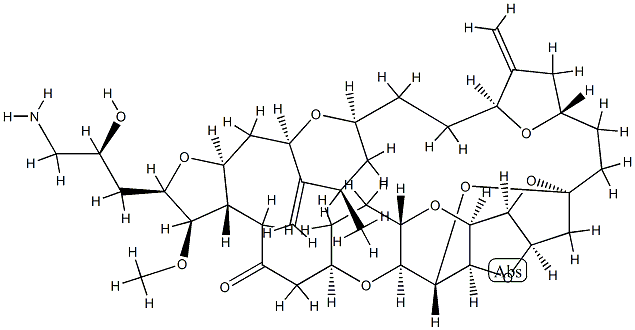
Eribulin synthesis
- Product Name:Eribulin
- CAS Number:253128-41-5
- Molecular formula:C40H59NO11
- Molecular Weight:729.9

30566-92-8
117 suppliers
inquiry

253128-41-5
133 suppliers
inquiry
Yield:253128-41-5 88%
Reaction Conditions:
Stage #1: C45H67NO13with 2,6-dimethylpyridine;trimethylsilyl trifluoromethanesulfonate in dichloromethane at 0 - 20; for 2.5 h;
Stage #2: with potassium carbonate in methanol at 20; for 2 h;
Steps:
4.l; 20 EXAMPLE 20 : Preparation of Eribulin
EXAMPLE 20 : Preparation of Eribulin Compound lj (133 mg, 160 μηιοΙ, 1.0 eq) was dissolved in anhydrous dichloromethane (20 mL) and cooled to 0 °C. To this solution was sequentially added 2,6-lutidine (0.09 mL, 0.8 mmol, 5.0 eq), and TMSOTf (0.12 mL, 0.64 mmol, 4.0 eq) and the cooling bath was removed. The reaction was stirred at room temperature for 1.5 hours and another portion of 2,6-lutidine (5.0 eq) and TMSOTf (4.0 eq) were added at room temperature. The reaction was further stirred for 1 hour and quenched with water (10 mL) . The layers were separated and the organic phase was washed with additional water (2x 10 mL), brine (10 m L), dried over MgS04 and concentrated under reduced pressure. The residue was dissolved in MeOH (10 mL), a catalytic amount of K2C03 was added at room temperature and the resulting mixture was stirred for 2 hours. The reaction was diluted with dichloromethane and quenched with water (10 mL) . The layers were separated and the aqueous phase was further extracted with dichloromethane (5 x 10 mL) The combined organic layers were washed with brine (20 mL), dried over MgS04, filtered and concentrated. The residue was dissolved in dichloromethane and purified by column chromatography on silica gel, using 1 : 9 MeOH : CH2CI2 to 1 : 9 : 90 NH4OH : MeOH : CH2CI2 as eluent. The product was afforded as a white amorphous solid (103 mg, 88%)
References:
WO2015/70,2015,A1 Location in patent:Paragraph 0059; 00117; 00118

253128-17-5
4 suppliers
inquiry

253128-41-5
133 suppliers
inquiry

253128-15-3
21 suppliers
inquiry

253128-41-5
133 suppliers
inquiry
![11,15:18,21:24,28-Triepoxy-7,9-ethano-12,15-methano-9H,15H-furo[3,2-i]furo[2',3':5,6]pyrano[4,3-b][1,4]dioxacyclopentacosin-5(4H)-one, hexacosahydro-2-[(2S)-2-hydroxy-3-[(methylsulfonyl)oxy]propyl]-3-methoxy-26-methyl-20,27-bis(methylene)-, (2R,3R,3aS,7R,8aS,9S,10aR,11S,12R,13aR,13bS,15S,18S,21S,24S...](/CAS/20211123/GIF/253128-19-7.gif)
253128-19-7
1 suppliers
inquiry

253128-41-5
133 suppliers
inquiry
![11,15:18,21:24,28-Triepoxy-7,9-ethano-12,15-methano-9H,15H-furo[3,2-i]furo[2',3':5,6]pyrano[4,3-b][1,4]dioxacyclopentacosin-5(4H)-one, hexacosahydro-3-methoxy-26-methyl-20,27-bis(methylene)-2-[(2S)-2-oxiranylmethyl]-, (2R,3R,3aS,7R,8aS,9S,10aR,11S,12R,13aR,13bS,15S,18S,21S,24S,26R,28R,29aS)-](/CAS/20211123/GIF/1190395-92-6.gif)
1190395-92-6
1 suppliers
inquiry

253128-41-5
133 suppliers
inquiry