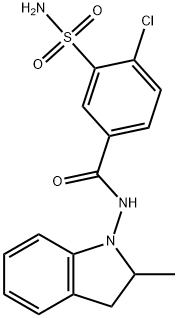
Indapamide synthesis
- Product Name:Indapamide
- CAS Number:26807-65-8
- Molecular formula:C16H16ClN3O3S
- Molecular Weight:365.83
1205-30-7
293 suppliers
$6.00/5g
6872-06-6
266 suppliers
$5.00/5g

26807-65-8
431 suppliers
$12.00/1mg
Yield:26807-65-8 95.5%
Reaction Conditions:
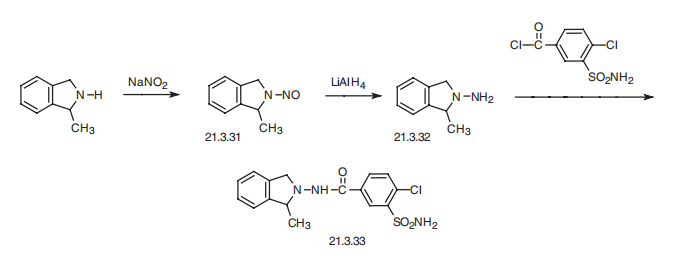
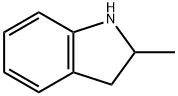
Stage #1:2-methyl indoline with triethylamine;hydroxylamine-O-sulfonic acid at 10;
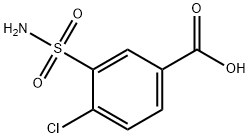
Stage #2:4-chloro-3-sulphamoylbenzoic acid with dicyclohexyl-carbodiimide at 15; for 6 h;Reagent/catalyst;
Steps:
1-4 Example 1
Add 50ml of hydroxylamine-O-sulfonic acid solvent to the reaction flask, add 17g of 2,3-dihydro-2-methyl-1H-indole, add 260ml of DMI solvent, and add 15ml of triethylamine dropwise with stirring, Cool the temperature to 10 °C, add 16g 4-chloro-3-sulfabenzoic acid, add 18ml N,N-dicyclohexylcarbodiimide, control the reaction temperature at 15 °C, the reaction time is 6 hours,After the reaction, the insoluble matter was filtered off, and isopropanol-water solvent was added for recrystallization to obtain indapamide product with a yield of 95.5%.
References:
Tianjin He Zhi Pharmaceutical Group Co., Ltd.;Cai Zhihe CN112142643, 2020, A Location in patent:Paragraph 0042-0049

63968-75-2
71 suppliers
$80.00/1 mg

26807-65-8
431 suppliers
$12.00/1mg

1205-30-7
293 suppliers
$6.00/5g
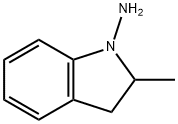
31529-46-1
103 suppliers
$55.00/10 g

26807-65-8
431 suppliers
$12.00/1mg