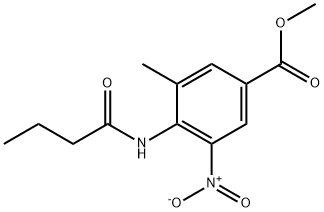
Methyl 4-(butyrylamino)-3-methyl-5-nitrobenzoate synthesis
- Product Name:Methyl 4-(butyrylamino)-3-methyl-5-nitrobenzoate
- CAS Number:152628-01-8
- Molecular formula:C13H16N2O5
- Molecular Weight:280.28
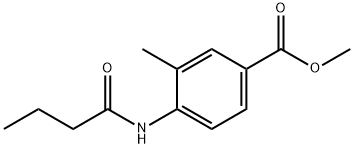
301533-59-5
97 suppliers
$10.00/250mg

152628-01-8
168 suppliers
inquiry
Yield:152628-01-8 86%
Reaction Conditions:
with sulfuric acid;nitric acid in dichloromethane at 5 - 32; for 0.4 h;Solvent;Temperature;
Steps:
1-12
The structure of the dedicated reaction device used in this embodiment is shown in Figure 1. The reactor pipe length is 25m, the diameter is 3mm, and the pipe material is Teflon. First turn on the pre-cooling pipeline of the first semiconductor temperature control box 7, Set the temperature at 10°C, turn on the second semiconductor temperature control box 13, Set the temperature to 25; Then store a sufficient amount of fuming nitric acid in the fuming nitric acid storage tank 1, Store a sufficient amount of 98% sulfuric acid in the concentrated sulfuric acid storage tank 2, Dissolve 23.5 grams (0.1 mol) of methyl 3-methyl-4-butyramidobenzoate in 200 mL of dichloromethane and store it in the storage tank 8. Enough ice water at 5°C is stored in the quenching agent storage tank 15. Start the first metering pump 3 and the second metering pump 5, Control the flow of the two pumps so that the molar equivalent ratio of nitric acid and sulfuric acid is 3:1, The flow rate is controlled to keep the temperature of the mixed acid flowing out of the first pipeline reactor 6 at about 15°C. When the mixed acid is about to reach the second high-efficiency mixer 11, turn on the third metering pump 9, And control the flow so that the molar equivalent ratio of raw materials and mixed acid is 1:1.8, Control the reaction temperature at 30-32°C. When the reaction liquid is about to reach the third high-efficiency mixer 17, Turn on the fourth metering pump 16, Make the volume ratio of quencher and reaction solution 1:1, The temperature of the reaction solution after quenching is measured to be 34-35°C. The entire reaction and quenching process took 24 minutes. Collect the reaction liquid with a separator tank 19, Adjust the first throttle valve 20 and the second throttle valve 21, In this example, the water phase is separated from the upper layer of the liquid separation tank into the first receiving tank 22, The organic phase is collected in the second receiving tank 23 from the lower layer of the liquid separation tank. The organic phase is subsequently washed with 10% sodium carbonate, Dry with anhydrous sodium sulfate, Then the solid obtained by concentration is recrystallized with methanol: water (40:1), The filter cake was dried to obtain 24.1 grams of pure methyl 3-methyl-4-butyramido-5-nitrobenzoate. The yield was 86%,
References:
Shanghai Bei Ka Pharmaceutical Co., Ltd.;Kang Litao;Li Qian;Yang Shiqiong;Li Fajing CN111925285, 2020, A Location in patent:Paragraph 0044-0090
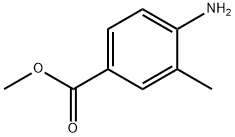
18595-14-7
298 suppliers
$8.00/5g

152628-01-8
168 suppliers
inquiry
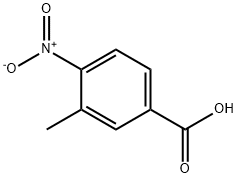
3113-71-1
460 suppliers
$6.00/25g

152628-01-8
168 suppliers
inquiry
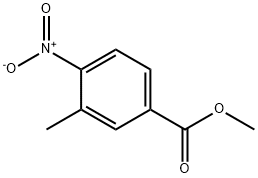
24078-21-5
239 suppliers
$6.00/5g

152628-01-8
168 suppliers
inquiry