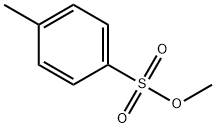
Methyl p-toluenesulfonate synthesis
- Product Name:Methyl p-toluenesulfonate
- CAS Number:80-48-8
- Molecular formula:C8H10O3S
- Molecular Weight:186.23
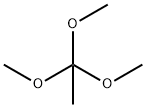
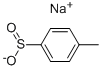
1445-45-0
338 suppliers
$10.00/5mL
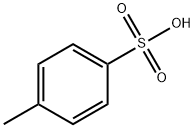
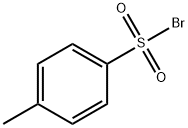
104-15-4
712 suppliers
$133.00/500g

80-48-8
441 suppliers
$5.00/25g
Yield:80-48-8 100%
Reaction Conditions:
at 20; for 0.5 h;
Steps:
1
Methyl tosylate alkylation agent was prepared p-toluenesulfonic acid with trimethyl orthoester at room temperature.
The reaction was complete in 30 minutes with 100% yield.
12.8 g of methyl tosylate was reacted with 2.0 g glycerol in aqueous NaOH 8 mL 40% aq. NaOH) in a small scale vessel for 3 hours at 100° C.
Trimethoxy glycerol ether (TMG-E) was obtained in near quantitative yield as a volatile clear solvent.
The gas chromatograph (top) and mass spectrum (bottom) for the trimethoxy glycerol ether is shown in .
References:
US8722943,2014,B1 Location in patent:Page/Page column 6
103-19-5
146 suppliers
$19.00/5g

80-48-8
441 suppliers
$5.00/25g