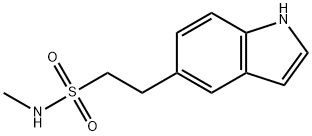
N-Methyl-1H-Indole-5-EthaneSulphonamide synthesis
- Product Name:N-Methyl-1H-Indole-5-EthaneSulphonamide
- CAS Number:98623-50-8
- Molecular formula:C11H14N2O2S
- Molecular Weight:238.31

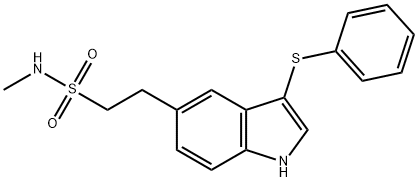
1204192-94-8
0 suppliers
inquiry

98623-50-8
80 suppliers
inquiry
Yield:98623-50-8 95%
Reaction Conditions:
with hydrogen at 40 - 45; under 2250.23 - 3000.3 Torr; for 10 h;Autoclave;
Steps:
3.4. 2-(1H-Indol-5-yl)-ethanesulfonic acidmethylamide(1)
2-(3-Phenylsulf-anyl-1H-indol-5-yl)-ethanesulfonic acidmethylamide (14) (5 g, 0.014 mol) was hydrogenated inethanol: water (7:3) (40 ml) in a autoclave at 40-45oC under3-4 kg/cm2 hydrogen pressure for 10 hrs. After completionof the reaction the reaction mass was cooled to room temperatureand was filtered to remove the catalyst. The filtratewas distilled out to get the product as a white solid whichwas re-crystallised with isopropyl alcohol to give 2-(1HIndol-5-yl)-ethanesulfonic acid methylamide (1). Yield: 3.2g (95%). Mp 260oC. Molecular formula: C11H14N2O2S; Calc.C, 55.46; H, 5.88; N, 11.76, Found: C, 55.34; H, 5.90; N,11.70; IR(KBr, νmax, cm-1): 3415(-NH str of sulfonamide),3289(-NH str of indole);1H NMR(400 MHz, δ ppm, DMSO):2.68(s, 3H,C12-H), 3.14(dd, 2H, J = 4.12 Hz, J = 5.92 Hz,C10-H), 3.27(dd, 2H, J = 4.16 Hz, J = 5.20 Hz, C11-H),6.39(t, 1H, -NH sulfonamide), 6.44(s, 1H, ArC3-H), 6.97(dd,1H, J = 1.40 Hz, J = 1.44 Hz, ArC6-H), 7.19(t, 1H, ArC2-H),7.35(d, 1H, J = 8.28 Hz, ArC7-H), 7.40(s, 1H, ArC4-H),10.34(s, 1H, -NH indole); 13C NMR (400 MHz, δ ppm,DMSO):29.12(C-12),30.00(C-10), 52.65(C-11), 101.29(C-3), 111.82(C-7), 119.72(C-6), 121.91(C-4), 125.44(C-2),128.29(C-9), 128.78(C-8), 135.12(C-5),199.17(C-4); MS(m/z): 237.00[M-1]-.
References:
Behera, Ajaya Kumar;Majumdar, Poulomi;Mohanta, Prajna Parimita;Mishra, Sushanta Kumar [Letters in Organic Chemistry,2018,vol. 15,# 4,p. 265 - 269]

1305334-90-0
6 suppliers
inquiry

98623-50-8
80 suppliers
inquiry
![1H-Indole-2-carboxylic acid, 5-[2-[(methylamino)sulfonyl]ethyl]-](/CAS/20210305/GIF/1025797-51-6.gif)
1025797-51-6
2 suppliers
inquiry

98623-50-8
80 suppliers
inquiry
![Acetamide, N-[4-[2-[(methylamino)sulfonyl]ethyl]-2-[2-(trimethylsilyl)ethynyl]phenyl]-](/CAS/20210305/GIF/1268265-95-7.gif)
1268265-95-7
1 suppliers
inquiry

98623-50-8
80 suppliers
inquiry
![Acetamide, N-[2-ethynyl-4-[2-[(methylamino)sulfonyl]ethyl]phenyl]-](/CAS/20210305/GIF/1268265-99-1.gif)
1268265-99-1
1 suppliers
inquiry

98623-50-8
80 suppliers
inquiry