
P-AZIDOACETOPHENONE synthesis
- Product Name:P-AZIDOACETOPHENONE
- CAS Number:20062-24-2
- Molecular formula:C8H7N3O
- Molecular Weight:161.16
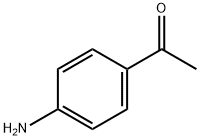
99-92-3
510 suppliers
$6.00/10g

20062-24-2
22 suppliers
inquiry
Yield:20062-24-2 95%
Reaction Conditions:
Stage #1: 4-Aminoacetophenonewith hydrogenchloride in water at 0 - 5; for 0.166667 h;
Stage #2: with 1-methyl-3-(2-[2-(1-methyl-1H-imidazol-3-ium-3-yl)ethyloxy]ethyl)-1H-imidazol-3-ium dinitrite in water; for 0.166667 h;
Stage #3: with sodium azide in water at 20; for 0.166667 h;Ionic liquid;
Steps:
General procedures for the conversion of aryl amines to aryl azides
General procedure: Aniline derivative (2 mmol) was dissolved in 1 ml of 37 % HCl. The mixture was stirred at 0-5 C for 10 min, until the ammonium salt was formed and the aromatic amine disappeared. The progress of the reaction was monitored by TLC [eluent: n-hexane/ethyl acetate (90:5)]. Nitrite ionic liquid (1 mmol) was added to theaniline solution. The reaction mixture was ground mildly for 5-10 min, the diazonium salt was formed. Then, NaN3 (5 mmol, 0.326 g) was added to the mixture and stirring continued for 6-25 min at room temperature (Table 2). The mixture was filtered and washed with distilled water (3 x 8 ml) and ethyl acetate (EtOAc, 15 ml). The residue was extracted with EtOAc (3 9 10 ml) and the combined organic layer was washed with 5 % aqueous solution of NaOH (12 ml) and then it was dried over anhydrous Na2SO4 . The solvent was evaporated to afford aryl azides.
References:
Eshghi, Hossein;Bakavoli, Mehdi;Ghasemzadeh, Marjan [Research on Chemical Intermediates,2013,vol. 41,# 6,p. 3999 - 4007]
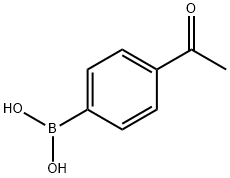
149104-90-5
298 suppliers
$10.00/250mg

20062-24-2
22 suppliers
inquiry
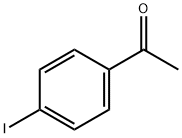
13329-40-3
266 suppliers
$6.00/5g

20062-24-2
22 suppliers
inquiry
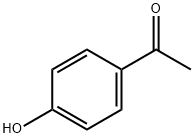
99-93-4
822 suppliers
$5.00/25g

20062-24-2
22 suppliers
inquiry

171364-81-1
94 suppliers
$17.00/250mg

20062-24-2
22 suppliers
inquiry