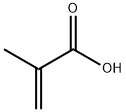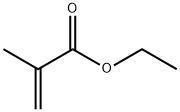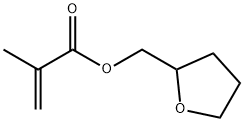
Tetrahydrofurfuryl methacrylate synthesis
- Product Name:Tetrahydrofurfuryl methacrylate
- CAS Number:2455-24-5
- Molecular formula:C9H14O3
- Molecular Weight:170.21
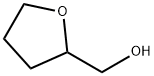
97-99-4
419 suppliers
$14.00/5g
80-62-6
631 suppliers
$18.00/25mL

2455-24-5
225 suppliers
$15.02/25ML
Yield:2455-24-5 95%
Reaction Conditions:
with 4-acetylamino-2,2,6,6-tetramethyl-1-piperidinoxy;di(n-butyl)tin oxide in 2-Methylpentane;cyclohexane at 85 - 100; for 12 h;Temperature;
Steps:
VII-5
To a 2 L four-necked glass flask were attached a 20-stage Oldershaw, a reflux condenser and an air introductiontube, and the flask was charged with 616 g (7.0 moles) of tetrahydrofurfuryl alcohol, 911 g (9.1 moles) of methyl methacrylate,0.6 g of 4-acetamino-2,2,6,6-tetramethylpiperidine-N-oxyl, 6.4 g of dibutyltin oxide, 30 g of isohexane, and 70g of cyclohexane. While a nitrogen gas containing 8% by volume of oxygen gas was blown into the flask at a flow rateof 20 mL/min to the flask, the contents in the flask were heated to 85 to 100°C, and a transesterification reaction wasstarted under the atmospheric pressure. The reflux ratio in the tower top was adjusted so that the top temperature of the distillation tower was kept at45 to 46°C, an azeotropic temperature of methyl alcohol as a by-product and isohexane as a reaction solvent. Thecondensate distilled out of the system from the tower top was cooled to 20°C and led to a decanter. Of the two layers separated in the decanter, the upper layer, which was a layer containing mainly isohexane,was refluxed to the tenth stage from the bottom of the distillation tower. The lower layer containing mainly methyl alcoholwas continuously taken out so that the separation interface in the decanter was kept constant. Since the lower layertaken out contained isohexane besides methyl alcohol, in order to prevent decrease of isohexane in the reaction system,the reaction temperature was adjusted to 85 to 100°C while isohexane in the same amount as that contained in thetaken lower layer was added to the reaction system. The transesterification reaction of methyl acrylate and tetrahydrofurfurylalcohol was completed within about 12 hours from the start of the reaction. The amount of the generated tetrahydrofurfuryl methacrylate was 1039 g (yield: 95%) and the total concentrationof Michael addition products in the reaction solution was not more than 0.1% by mass. Further, the lower layer containingmainly methyl alcohol and taken out at the time of the reaction contained 57.0% by mass of methyl alcohol, 0.5% bymass of cyclohexane, and 42.5% by mass of isohexane, and the amount of methyl methacrylate was not more than thedetection limit (detection limit: 10 ppm). Next, by using the distillation tower having a 10-stage Oldershaw, the lower layer was distilled. When a fractionwith a boiling point of 45 to 50°C was taken out from the tower top of the distillation tower, the fraction contained 82%by weight of isohexane, 17.6% by weight of methyl alcohol, and 0.4% by mass of cyclohexane. This fraction could beused as a reaction solvent. The remaining liquid in the flask contained 99.6% by weight of methyl alcohol, 0.3% by weightof isohexane, and 0.1% by weight of cyclohexane.
References:
Osaka Organic Chemical Ind., Ltd.;SUGIURA, Toyoyuki;OKAMURA, Kohei;ITO, Keisuke;KAWAKAMI, Naohiko;MATSUMOTO, Shigeaki;MATSUNO, Masayoshi EP2860170, 2015, A1 Location in patent:Paragraph 0635; 0636; 0637; 0638; 0639