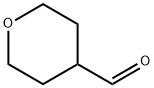
Tetrahydropyran-4-carbaldehyde synthesis
- Product Name:Tetrahydropyran-4-carbaldehyde
- CAS Number:50675-18-8
- Molecular formula:C6H10O2
- Molecular Weight:114.14
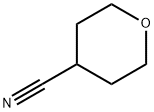
4295-99-2
169 suppliers
inquiry

50675-18-8
204 suppliers
$15.00/100mg
Yield:50675-18-8 80%
Reaction Conditions:
with diisobutylaluminium hydride in toluene at -5 - 25;Inert atmosphere;
Steps:
Tetrahydropyran-4-carbaldehyde (6)
[0169] Dry toluene (500 g) was charged into a dried reactor under nitrogen and tetrahydropyran-4-carbonitrile 5 (100 g, 0.90 mol) was added. The resulting solution was cooled to -5°C to 5°C and a solution of DIBAL-H in toluene (1.0M, 800 g, 1.0 mol) was added dropwise at -5°C to 5°C and the resulting reaction mxture was stirred at this temperature for 1-2 hrs. The reaction mixture was then warmed to 20-25°C and stirred at this temperature for 1-2 hrs. Subsequently the reaction mixture was cooled to -5°C - 5°C and quenched by the dropwise addition of a solution of AcOH (195 g) in toluene (180 g) at -5° - 5°C (caution: exothermic, gas release). A 25% solution of sodium tartrate tetrahydrate (1000 g) was added slowly at -5°C - 5°C (caution:exothermic, gas release). The reaction mixture was allowed to warm to 20-25°C and stirred at this temperature for 8-16 hrs. The layers were separated and the aqueous layer was extracted twice with EtOAc (900 mL each) at 20-25°C. The two EtOAc extracts were combined with the original organic phase and the combined organic phases were evaporated in vacuo to about 100-200 g to yield the product 6. The product was generally isolated in 50-80% yield.
References:
IMARA, INC.;SVENSTRUP, Niels;ZHANG, Jun;SUN, Jikui;CHEN, Yuyin;KONG, Jianshe;MA, Rujian;ZHANG, Junhua;QIN, Liang;XIAO, Huanming;SUN, Jinxu;MENG, Xiao;SUN, Fenglai;ZHU, Jingyang WO2018/218104, 2018, A1 Location in patent:Paragraph 0154; 0169
14774-37-9
242 suppliers
$5.00/1g

50675-18-8
204 suppliers
$15.00/100mg

101765-10-0
5 suppliers
inquiry

50675-18-8
204 suppliers
$15.00/100mg

110238-91-0
207 suppliers
$13.00/5g

50675-18-8
204 suppliers
$15.00/100mg
5337-03-1
297 suppliers
$10.00/1 g

50675-18-8
204 suppliers
$15.00/100mg