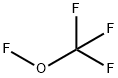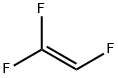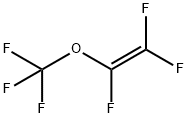
Trifluoromethyl trifluorovinyl ether synthesis
- Product Name:Trifluoromethyl trifluorovinyl ether
- CAS Number:1187-93-5
- Molecular formula:C3F6O
- Molecular Weight:166.02
Yield:1187-93-5 96%
Reaction Conditions:
with nickel at -30;Inert atmosphere;
Steps:
2.2a
Example2a: Two 27 ml micoreactors (first one made out of SiC, second one made of Ni) were in-stalled in series,the first microreactor was kept at room temperature (ambient temperature; about 25) by cooling, and the second microreactor was heated to 100, pressure was adjusted to 4 bar abs. by using a pressure valve installed at the gas exit at the cyclone. There was a cooler installed after these condmicroreactor to immediately cool down the reaction mixture to 0(cooler not shown in the Figure1) where the desired product PFMVE Is a gas and formed HF is in liquid state already. Also, after the second micro-reactor and after the cooler, the partially liquid reaction mixture wasfed into a cyclone with said pressure valve at gas exit (cyclone also not shown in the Figure 1). The liquid phase material of the cyclone (HF) moved into a storage tank for a re-use.The gas phase of the cyclone consisted mainly out of PFMVE (together with only some traces HF) and moves over a Swagelokh and valve (for further expanding to about normal pressure, e.g., at 1 atm) into the cooling trap by means of a deep pipe (a stainless ssteel cylinder equipped with deep pipe and a gas outlet); the cooling trap waskept at about-30..Before starting the reaction, the system is continuously floated with a He(helium) in-ert gas purge which purge was rapidly reduced once the feeding of raw materials has started and purge was stopped completely after reaching constant feed of the raw mate-rials into the reactor. A fast reduction of inert gas feed (purge) is essential as inert gas reduces sharply the heat exchange efficiency in both reactors. In to this reactor installation, floated with a He (helium) inert, CF3OF was fed out of a gas cylinder (out of gaseous phase) over a Bronkhorst mass flow controller toge the rwith gaseous trifluoroethylene out of another cylinder with 150 g (1.83mol/h)and over a Bronkhorst mass flow controller in a ratio of 1.05:1.0. Final distillation of the collected PFMVE was done in a pressure column made out of Hastelloy C4 (nickel alloy), at 5 bar abs. yielding 96% of PFMVE (99.9% GC-purity) based on trifluoroethylene starting material.
References:
WO2022/95625,2022,A1 Location in patent:Page/Page column 28
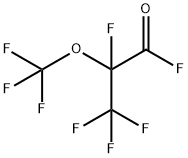
2927-83-5
2 suppliers
inquiry

1187-93-5
112 suppliers
$95.00/25g

94720-91-9
0 suppliers
inquiry

1187-93-5
112 suppliers
$95.00/25g
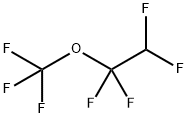
2356-61-8
2 suppliers
inquiry

1187-93-5
112 suppliers
$95.00/25g