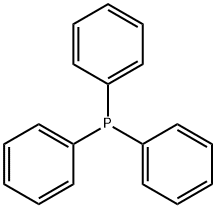
Triphenylphosphine hydrobromide synthesis
- Product Name:Triphenylphosphine hydrobromide
- CAS Number:6399-81-1
- Molecular formula:C18H16BrP
- Molecular Weight:343.2
603-35-0
698 suppliers
$5.00/5 g

6399-81-1
203 suppliers
$7.00/10g
Yield:6399-81-1 98%
Reaction Conditions:
with hydrogen bromide in 1,4-dioxane;water at 20 - 70; for 12 h;
Steps:
A solution of 48% aqueous hydrobromic acid (1mL, ca. 8.8 mmol) was added dropwise for 1 min to the stirring mixture oftriphenylphosphine (1.05 g, 4 mmol) in dioxane (8 mL) at room temperature, and warmed to70 °C. After the mixture was stirred at 70 °C for 12 h, the mixture was evaporated in vacuo.The residue was washed with ether, and the precipitate was dried in vacuo for 12 h at 40 °C togive 1a•HBr (1.34 g, 98%) as a white solid; 1H NMR (400 MHz, DMSO-d6, 25 C) δ (ppm):7.53-7.59 (m, 6H, ArH), 7.60-7.67 (m, 9H, ArH), 10.60 (brs, 1H, PH). 13C NMR (100 MHz,DMSO-d6, 25 °C) δ (ppm): 128.77 (d, J = 11.4 Hz, Ar), 131.48 (d, J = 9.6 Hz, Ar), 132.05 (d,J = 1.9 Hz, Ar), 132.70 (d, J = 102 Hz, Ar).
References:
Aoyagi, Naoto;Furusho, Yoshio;Endo, Takeshi [Tetrahedron Letters,2013,vol. 54,# 51,p. 7031 - 7034] Location in patent:supporting information

3968-92-1
44 suppliers
$95.09/5g

6399-81-1
203 suppliers
$7.00/10g