ε-Caprolactone-based craft copolymers: synthesis and applications in the biomedical field
Sep 15,2023
General Description
ε-Caprolactone-based graft copolymers are high-performance engineering plastics composed of polyester and polyether. They possess excellent mechanical properties, chemical resistance, low friction coefficient, and wear resistance. The synthesis involves copolymerizing a functionalized PCL chain with caprolactone, achieved through the modification of PCL using α-chloro-ε-caprolactone and sodium azide, followed by grafting alkyne-PEG via Huisgen CuAAC cycloaddition. With an amphiphilic structure, PCL-g-PEG finds applications in diverse areas. In the biomedical field, ε-caprolactone-based graft copolymers show promise in anti-tumor drug delivery systems. These copolymers enable controlled drug release, uniform distribution, prolonged degradation, biocompatibility, and non-toxicity. They can serve as carriers or adjuvants in vaccine preparation, enhancing stability, immunogenicity, and efficacy. The controlled release properties promote sustained antigen presentation and improved immune responses. Future research aims to explore the potential application of these copolymers in different fields, utilizing their unique properties to develop advanced drug delivery systems and vaccines.

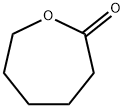
Figure 1. ε-Caprolactone
Synthesis
The synthesis of ε-caprolactone-based graft copolymers, particularly PCL-g-PEG, is commonly achieved through the copolymerization of a functionalized PCL chain with genuine caprolactone. One widely used method involves the preparation of a functionalized PCL by copolymerizing α-chloro-ε-caprolactone (αCl-CL) with CL. αCl-CL can be obtained by oxidizing α-chlorocyclohexanone with m-chloro-peroxybenzoic acid. The resulting P(αCl-CL-co-CL) is then modified to P(α-azido-CL-co-CL) by substituting the chlorine atom with sodium azide. Grafting of an alkyne-PEG onto poly(α-azido-CL-co-CL) is accomplished through a Huisgen CuAAC cycloaddition, resulting in the formation of PCL-g-PEG. This amphiphilic copolymer forms micelles, with the critical micelle concentration (CMC) and diameter dependent on the degree of PEG grafting. The amphiphilic structure of PCL-g-PEG, attributed to the hydrophilicity of the PEG segments and the hydrophobicity of the PCL chain, contributes to its main applications. It is worth noting that the term PCL-g-PEG is used generically for all copolymers, regardless of the linkage between the PCL chain and the PEG. Further research aims to explore the application of these copolymers in various fields, harnessing their unique properties. 1
Applications in the biomedical field
Anti-tumor drug delivery systems
ε-Caprolactone-based graft copolymers have shown great potential in the field of anti-tumor drug delivery systems. Poly-epsilon caprolactone and its derivative copolymers are biocompatible and biodegradable, making them ideal candidates for drug carriers. The controlled release of anti-tumor drugs is a major advantage of these systems, providing uniform drug distribution, prolonged degradation and release process, non-toxicity, and compatibility with body tissues. Poly-ε-Caprolactone has been approved by the US Food and Drug Administration, making it a promising platform for the design and fabrication of anti-tumor drug delivery systems with controllable drug release behaviors. Recent studies have focused on the application of poly-ε-Caprolactone-based materials in the controlled release of cancer therapy drugs. Various formulations of poly-ε-Caprolactone-based materials have been evaluated, leading to increased drug loading capacity, reduced leakage, prolonged release periods, and controllable release rates. Additionally, the development of environment-responsive delivery systems, such as pH-, thermo-, magnetic field-, and light-responsive drug carriers, has enhanced tumor cell targeting potential and decreased systemic toxicity. These advancements show great promise in the field of anti-tumor drug delivery, with potential for improved therapeutic outcomes and reduced side effects. 2
Vaccine preparation
ε-Caprolactone-based graft copolymers have emerged as promising materials for vaccine preparation. These copolymers, including poly-ε-Caprolactone (PCL) and its derivatives, exhibit excellent biocompatibility, biodegradability, and controlled release properties, making them suitable candidates for vaccine delivery systems. In vaccine preparation, these copolymers can serve as vaccine carriers or adjuvants, enhancing the stability, immunogenicity, and efficacy of vaccines. They can encapsulate antigens within their structure, protecting them from degradation and promoting sustained release, thus prolonging the immune response. The controlled release properties of ε-caprolactone-based graft copolymers enable a gradual and prolonged antigen presentation, leading to enhanced immune responses. The application of ε-caprolactone-based graft copolymers in vaccine preparation has shown promising results, offering new strategies for the development of safe and effective vaccines. Further research and development in this area hold great potential for improving vaccine delivery systems and enhancing immune responses. 3
Reference
1. Coudane J, Nottelet B, Mouton J, Garric X, Van Den Berghe H. Poly(ε-caprolactone)-Based Graft Copolymers: Synthesis Methods and Applications in the Biomedical Field: A Review. Molecules, 2022, 27(21):7339.
2. Sun Z, Duan R, Xing D, Pang X, Li Z, Chen X. Recent Advances in Application of Poly-Epsilon-Caprolactone and its Derivative Copolymers for Controlled Release of Anti-Tumor Drugs. Curr Cancer Drug Targets, 2017, 17(5):445-455.
3. Li P, Song H, Zhang H, et al. Engineering biodegradable guanidyl-decorated PEG-PCL nanoparticles as robust exogenous activators of DCs and antigen cross-presentation. Nanoscale, 2017, 9(36):13413-13418.
- Related articles
- Related Qustion
- Exploring ε-Caprolactone: A Versatile Monomer for the Chemical Industry Nov 5, 2024
ε-Caprolactone, a pivotal chemical in the realm of polymer science, is increasingly gaining attention for its broad utility and unique properties.
- ε-Caprolactone: Applications in Nanotechnology and its Toxicity Oct 23, 2024
ε-Caprolactone-modified polyethylenimine nanocarriers enhance siRNA delivery, demonstrating minimal toxicity and high biocompatibility, making them crucial for safe gene therapy.
- What is ε-Caprolactone?Uses_Chemical reaction_Safety Sep 30, 2020
ε-Caprolactone or simply caprolactone is a lactone (a cyclic ester) possessing a seven-membered ring. Its name is derived from caproic acid. This colorless liquid is miscible with most organic solvents and water.
ε-Caprolactone
502-44-3You may like
- 6-Hexanolactone
-

- $10.00 / 1KG
- 2025-11-14
- CAS:502-44-3
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 10 mt
- ε-Caprolactone
-
- $35.00 / 1kg
- 2025-09-25
- CAS:502-44-3
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: g-kg-tons, free sample is available
- 6-Hexanolactone
-

- $0.00 / 25KG
- 2025-09-08
- CAS:502-44-3
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 50000KG/month