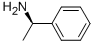(R)-(+)-1-Phenylethylamine: Role in Schizophrenia and its Biosynthesis Method
Jun 20,2024
General Description
The investigation of (R)-(+)-1-Phenylethylamine in schizophrenia reveals elevated urinary levels in individuals with the disorder, particularly in paranoid schizophrenics, suggesting a potential biomarker. However, studies on cerebrospinal fluid and plasma levels show mixed results, indicating complexity in its role across different biological matrices. In biosynthesis, certain bacterial strains like Enterococcus faecium can produce (R)-(+)-1-Phenylethylamine from phenylalanine through decarboxylation. Further research is needed to understand the clinical implications of (R)-(+)-1-Phenylethylamine in schizophrenia and its biosynthesis pathways in bacteria.

Figure 1. (R)-(+)-1-Phenylethylamine
Role in Schizophrenia
(R)-(+)-1-Phenylethylamine has been a significant point of investigation in the context of schizophrenia, revealing intriguing findings that might help understand the biochemical pathways of this complex disorder. Schizophrenia, characterized by symptoms such as delusions, hallucinations, and cognitive impairments, has been linked to various neurochemical imbalances, including those involving (R)-(+)-1-Phenylethylamine. Initial studies in the late 1960s and early 1970s by Fischer et al. and Heller and Fischer identified an increase in urinary excretion of (R)-(+)-1-Phenylethylamine in individuals with schizophrenia. These findings have been pivotal, as they suggest a potential biomarker for schizophrenia, particularly noting that increased levels of (R)-(+)-1-Phenylethylamine were more pronounced in paranoid schizophrenics. This specific observation has led to hypotheses that paranoid schizophrenia might have unique biochemical underpinnings, possibly associated with elevated (R)-(+)-1-Phenylethylamine levels. Further investigations using advanced analytical methods like gas chromatography-mass spectrometry have consistently supported the notion of elevated urinary (R)-(+)-1-Phenylethylamine levels in schizophrenic patients. These consistent findings across larger patient cohorts underscore the potential role of (R)-(+)-1-Phenylethylamine in the pathology of schizophrenia. Despite these significant findings in urinary excretion, studies examining (R)-(+)-1-Phenylethylamine levels in other bodily fluids like cerebrospinal fluid and plasma have presented mixed results. For instance, Beckmann et al. did not find significant differences in (R)-(+)-1-Phenylethylamine levels in the cerebrospinal fluid of schizophrenics compared to healthy controls, suggesting that the role of (R)-(+)-1-Phenylethylamine might be more complex and not uniformly expressed across different biological matrices. Furthermore, studies on plasma levels of (R)-(+)-1-Phenylethylamine also showed variable results, with some studies like those by Szymanski et al. reporting lower levels, especially in paranoid schizophrenics, while others found elevated levels. These discrepancies highlight the complexity of schizophrenia as a disorder and indicate that the role of (R)-(+)-1-Phenylethylamine might vary based on the biological context and methodological differences among studies. In summary, while (R)-(+)-1-Phenylethylamine has been consistently observed at increased levels in the urine of schizophrenic patients, its overall clinical application in schizophrenia requires further research to understand its role across different subtypes and fluid matrices. The elevated levels of (R)-(+)-1-Phenylethylamine particularly in paranoid schizophrenics suggest a potential subgroup-specific biomarker that could advance personalized treatment strategies. 1
Biosynthesis Method
(R)-(+)-1-Phenylethylamine is a biogenic amine that can be produced through biosynthesis pathways in certain bacterial strains, particularly Enterococcus faecium. The biosynthesis of PEA involves the decarboxylation of phenylalanine by a dual decarboxylase enzyme present in these bacteria. Studies conducted by Marcobal et al in 2004 and 2006 revealed the presence of a gene in E. faecium strains that encodes a dual decarboxylase enzyme capable of converting both phenylalanine and tyrosine into their corresponding amines. This enzyme exhibits activity towards phenylalanine, leading to the production of (R)-(+)-1-Phenylethylamine, alongside its known activity on tyrosine for tyramine production. Research by Beutling and Walter in 2002 demonstrated the simultaneous production of (R)-(+)-1-Phenylethylamine and tyramine by various enterococcal strains, notably E. faecalis and E. hirae. Similarly, findings by Aymerich et al in 2006 and Landete et al in 2007 highlighted the ability of certain Lactobacillus strains to produce (R)-(+)-1-Phenylethylamine in addition to tyramine. Further investigations by Gardini and colleagues in 2001 and 2008 underscored the production of PEA by E. faecalis strains, particularly in dry fermented sausages, where significant levels of (R)-(+)-1-Phenylethylamine were detected alongside tyramine. These studies elucidated the dual decarboxylase activity of the enzyme responsible for the conversion of phenylalanine to PEA. Overall, the biosynthesis of (R)-(+)-1-Phenylethylamine in bacteria such as Enterococcus species involves specific enzymatic pathways that lead to the production of this biogenic amine alongside other compounds like tyramine. 2
Reference
1. O'Reilly RL, Davis BA. Phenylethylamine and schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 1994; 18(1): 63-75.
2. Marcobal A, De las Rivas B, Landete JM, Tabera L, Muñoz R. Tyramine and phenylethylamine biosynthesis by food bacteria. Crit Rev Food Sci Nutr. 2012; 52(5): 448-467.
- Related articles
- Related Qustion
- How to use (R)-1-phenylethylamine correctly Apr 29, 2022
(R)-1-phenylethylamine is an organic compound with the molecular formula C8H11N.
p-Anisaldehyde is a colorless liquid with a strong aroma. It has sweet, floral and strong aniseed odor.....
Nov 13,2024Organic ChemistryXylitol, as a naturally occurring polyol, is widely used as a sweetener and functional additive in food industry, medicine, chemical industry and other fields.....
Oct 30,2024Organic Chemistry(R)-(+)-1-Phenylethylamine
3886-69-9You may like
(R)-(+)-1-Phenylethylamine manufacturers
- (R)-(+)-1-Phenylethylamine
-

- $99.00/ kg
- 2024-11-17
- CAS:3886-69-9
- Min. Order: 0.0010000000474974513kg
- Purity: 99%
- Supply Ability: 5000
- (R)-(+)-1-Phenylethylamine
-

- $180.00 / 1kg
- 2024-11-15
- CAS:3886-69-9
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 20ton
- (R)-(+)-1-Phenylethylamine
-

- $0.00 / 200KG
- 2024-11-15
- CAS:3886-69-9
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 2000MT