Avatrombopag Maleate: Preclinical Pharmacology, Pharmacokinetics and Metabolism
Mar 28,2024
General Description
Avatrombopag maleate, a potent TPO receptor activator, exhibits promising preclinical pharmacology and pharmacokinetic properties. In preclinical studies, it demonstrated a concentration-dependent increase in hematopoietic progenitor cells, megakaryocyte precursors, and megakaryocytes, with synergistic effects when combined with recombinant human TPO. Additionally, in NOD/SCID mice transplanted with human fetal liver CD34+ cells, daily oral Avatrombopag maleate administration led to dose-dependent human platelet generation without affecting murine platelet counts, highlighting its selective action on human cells. Regarding pharmacokinetics, Avatrombopag maleate's slow absorption, hepatic metabolism via CYP enzymes, and predominantly fecal excretion with minimal renal elimination were noted. Genetic variations in CYP2C9 may impact drug metabolism among populations. The linear correlation between plasma concentration and platelet counts, influenced by endogenous TPO levels and albumin concentration, underscores its therapeutic potential for platelet disorders. Overall, Avatrombopag maleate's preclinical profile supports its development as a promising agent for stimulating platelet production with a favorable safety and efficacy profile that warrants further clinical investigation, especially in immune thrombocytopenia patients.

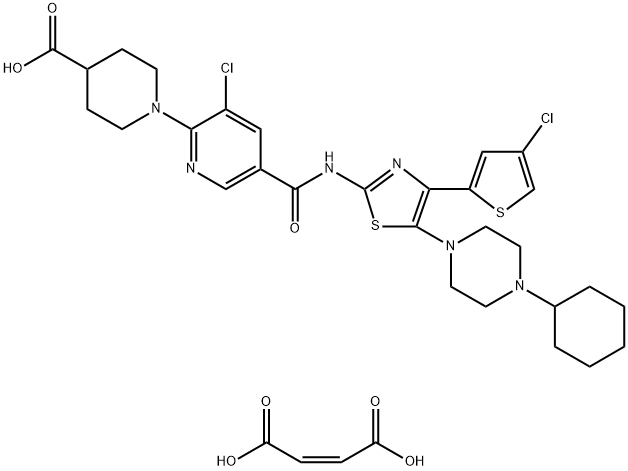
Figure 1. Avatrombopag maleate
Preclinical Pharmacology
Avatrombopag maleate, a potent small molecule activator of TPO receptors specifically in human and chimpanzee cells, demonstrates promising preclinical pharmacology. In a study investigating its effects on hematopoietic progenitor cells, megakaryocyte precursors, and megakaryocytes, avatrombopag exhibited a concentration-dependent increase in these cell populations when used alone or in combination with recombinant human TPO. Notably, the combination of Avatrombopag maleate and recombinant human TPO showed a synergistic effect, leading to a substantial enhancement in megakaryocyte production compared to TPO alone. Further preclinical research involving Avatrombopag maleate in nonobese diabetic/severe combined immunodeficiency (NOD/SCID) mice transplanted with human fetal liver CD34+ cells demonstrated that daily oral administration of avatrombopag resulted in a dose-dependent increase in human platelet generation without affecting murine platelet counts. This indicates the potential of Avatrombopag maleate as a therapeutic agent for stimulating platelet production specifically in human cells while maintaining the balance of platelet levels in animal models. Overall, the preclinical pharmacology of Avatrombopag maleate highlights its selective activation of TPO receptors, synergistic effects with recombinant human TPO, and its ability to enhance megakaryocyte and platelet production, showcasing its potential as a promising candidate for further development in the treatment of conditions related to platelet disorders. 1
Pharmacokinetics and Metabolism
Avatrombopag maleate, a novel TPO receptor activator, has undergone extensive studies focusing on its pharmacokinetics and metabolism. The key findings regarding Avatrombopag maleate's pharmacokinetic profile can be summarized as follows: Firstly, the absorption of avatrombopag is characterized by slow uptake due to its poor aqueous solubility. Peak plasma concentrations (Cmax) typically occur 5-8 hours post-dose, with absorption unaffected by food intake, distinguishing it from eltrombopag. Secondly, the distribution of Avatrombopag maleate shows an increase in volume with body weight, although this does not warrant significant clinical adjustments based on weight. Thirdly, Avatrombopag maleate is predominantly metabolized in the liver by cytochrome P450 enzymes, primarily CYP2C9 and CYP3A. Notably, a major metabolite, 4-hydroxy avatrombopag, does not appear in plasma. Regarding elimination, avatrombopag and its metabolites are mainly excreted via feces, with a minor fraction eliminated in urine. Approximately 34% of the drug is excreted unchanged. Furthermore, pharmacogenomic investigations have identified genetic variations in CYP2C9 that can influence avatrombopag metabolism, potentially leading to varying drug clearance and exposure levels across different populations. Lastly, the pharmacokinetic/pharmacodynamic relationship of avatrombopag demonstrates a linear correlation between plasma drug concentration and platelet counts. This relationship is influenced by factors like endogenous serum TPO levels and albumin concentration. In conclusion, the pharmacokinetic and metabolic characteristics of Avatrombopag maleate underscore its potential as a promising therapeutic option for platelet disorders. Further clinical research is essential to delve into its efficacy and safety profiles in patient cohorts, particularly individuals with immune thrombocytopenia. 2
Reference
1. Fukushima-Shintani M, Suzuki K, Iwatsuki Y, et al. AKR-501 (YM477) in combination with thrombopoietin enhances human megakaryocytopoiesis. Exp Hematol. 2008;36(10):1337-1342.
2. Al-Samkari H. Avatrombopag maleate for the treatment of periprocedural thrombocytopenia in patients with chronic liver disease. Drugs Today (Barc). 2018;54(11):647-655.
- Related articles
- Related Qustion
- Avatrombopag maleate: Preparation, Appication, Bioactivity Dec 28, 2022
Avatrombopag maleate is indicated for the treatment of thrombocytopenia in adult CLD patients scheduled to undergo medical or dental surgery.
Pal-Tripeptide-1 enhances collagen, skin elasticity, and reduces wrinkles effectively, with superior antioxidant properties, making it a key anti-aging skincare ingredient.....
Nov 13,2024APIBivalirudin, a thrombin inhibitor, offers effective anticoagulation in invasive cardiology, reducing clot risks with reversible binding, especially beneficial in various cardiovascular procedures.....
Nov 8,2024APIAvatrombopag maleate
677007-74-8You may like
Avatrombopag maleate manufacturers
- Avatrombopag Maleate
-

- $0.00 / 1g
- 2024-11-18
- CAS:677007-74-8
- Min. Order: 1g
- Purity: More Than 99%
- Supply Ability: 50kg/Month
- Avatrombopag maleate
-
- $1.00 / 1kg
- 2024-11-18
- CAS:677007-74-8
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 200kg
- Avatrombopag maleate
-

- $2500.00 / 100mg
- 2024-10-28
- CAS:677007-74-8
- Min. Order:
- Purity:
- Supply Ability: 10g