Chemical Reactivity of 2,5-Dihydrothiophene
Jan 28,2022
The parent 2,5-dihydrothiophene is a liquid with a bp of 122°C and an mp of –50.3°C. It is soluble in most of the organic solvents.

Chemical Reactivity
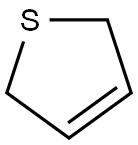
1.Oxidation 2,5-Dihydrothiophene oxidized with 30% H2O2 at low temperature provided 2,5-dihydrothiophene sulfoxide in 48% yields. However, hydrogen peroxide oxidation in acetic acid at 20°C for 24 h and thereafter boiling for 3 h gave 2,5-dihydrothiophene-1,1-dioxide.
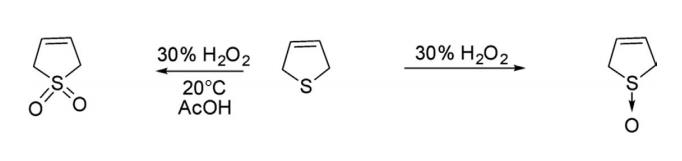
2. Reduction Hydrogenation of 2,5-dihydrothiophene over Pd/C in ethanol at 20°C for 12 min produced tetrahydrothiophene.
3. Addition of Thiols Addition of thiols to 2,5-dihydrothiophenes takes place at high temperature in an autoclave at 200°C to yield 3-alkylthiotetrahydrothiophene.

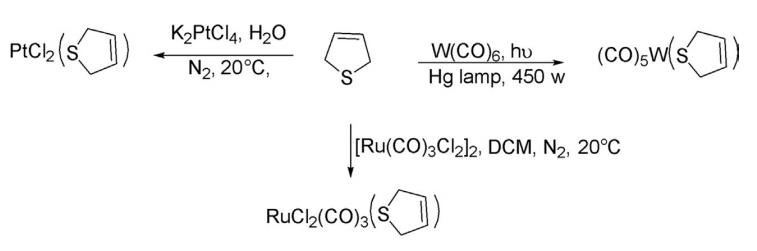
4. Reaction With Metal Complexes 2,5-Dihydrothiophene readily forms complexes on reaction with W(CO)6 , [Ru(CO)3 Cl2]2 , and K2 PtCl4 at room temperature to form various complexes.
5. Interconversion 2,5-Dihydrothiophene on heating at 300°C in the presence Re/Al2O3 isomerized to 2,3-dihydrothiophene in 21.8% yield.

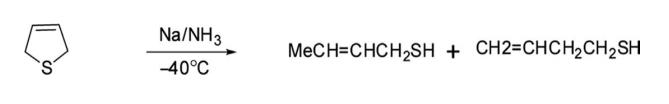
6. Ring-Opening Reaction Reduction of 2,5-dihydrothiophene with sodium in liquid ammonia in methanol gave a mixture of 2-butanethiol and 3-butanethiol as ring-opened compounds.
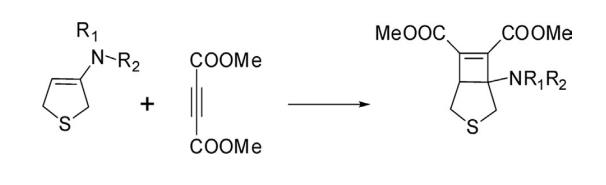
7. Cycloaddition Reactions 2,5-Dihydrothiophene having an enamine structural feature undergoes cycloaddition reactions with active dienophile. Thus a reaction of 3-dialkylamino-2,5-dihydrothiophene with dimethyl acetylene dicarboxylate gave a bicyclic compound, dimethyl 1-(dialkylamino)-3-thiabicyclo [3.2.0]hept-6-ene-6,7-dicarboxylate.
- Related articles
- Related Qustion
- Synthesis of 2,5-Dihydrothiophene Jan 27, 2022
2,5-Dihydrothiophene is a nonaromatic, partially saturated, sulfur-containing five-membered heterocycle, comprised of four carbon atoms and one sulfur atom with a double bond between the C3 and C4 carbon atoms. It is also known as 3-thiolen
Sodium acetate trihydrate is a promising heat storage material (m.p:58°C, heat of fusion, AHf~s:252 kJ/ kg (60.3 cal/g)....
Jan 28,2022APIBenzo[c]thiophene, also known as isobenzothiophene, is a bicyclic heteroaromatic sulfur heterocycle, constituted by fusion of benzene with the“c” site (3,4-positions) of the thiophene ring. It is less stable than benzo[b]thiophene because t....
Jan 28,2022Pharmaceutical intermediates2,5-Dihydrothiophene
1708-32-3You may like
- The uses of 5-Aminolevulinic acid hydrochloride
Jul 15, 2024
- What is EVA used for?
Jul 10, 2024
- Is it safe to use Ethylhexylglycerin
Jul 3, 2024