Ethylenediaminetetraacetic acid(EDTA)– Physical Properties and Applications
Apr 17,2020
Physical Properties of EDTA
The chemical names of ethylenediaminetetraacetic acid include Ethylenediamine tetraacetic acid, (Ethylenedinitrilo) tetraacetic acid, Edetic acid and EDTA. EDTA is white powder, which is soluble in sodium hydroxide, sodium carbonate and ammonia solution, and 160 parts of boiling water, and slightly soluble in cold water, insoluble in ethanol and general organic solvents. It can form extremely stable water-soluble complexes with alkali metals, rare earth elements and transition metals. EDTA decompose above its melting point 240°C.
Applications
Ethylenediaminetetraacetic acid (EDTA) is a substituted diamine widely used in domestic and industrial applications. EDTA chelate metal divalent cations, such as calcium, magnesium, zinc, copper, and manganese, to form metal-EDTA complexes. It is suitable for cleaning products and detergent formulations. In the paper and pulp industry, EDTA reduces the adverse effects of metal ions on bleaching. As an antibacterial agent, it can remove calcium and magnesium divalent cations in the outer membrane and cause the loss of membrane lipopolysaccharide, making bacteria sensitive to bactericides. Free EDTA has an adverse effect on mammalian reproduction and development. EDTA can sequester ions in sediments that cause atherosclerosis, cancer and heart disease. It reduces free radical reactions and oxidation processes, which helps to overcome cell membrane damage. It can bind to calcium and reduce the risk of developing hypercalcemia in cancer patients. The transmembrane ethylenediaminetetraacetic acid gradient method facilitates drug delivery, thereby enhancing drug retention and therapeutic effects and reducing levels of cytotoxicity.

Fig 1. The application of EDTA in drug delivery systems
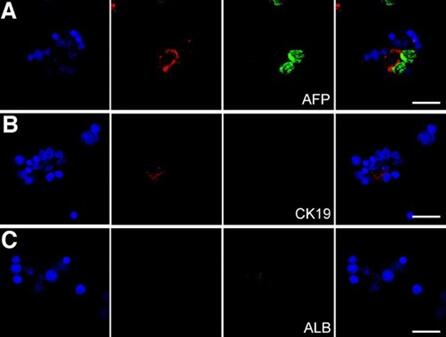
Fig 2. Images of EDTA used as Cell sorting analysis
The applications of Ethylenediaminetetraacetic acid (EDTA) have been reported as following [1-7]:
• Complexing agent. Ethylenediaminetetraacetic acid (EDTA) is a hydrophilic gold chelator that converts metal ions into inactive, cyclized metal complexes. Therefore, it can be used in industry to solve metal pollutants.
• Blood anticoagulant. Ethylenediaminetetraacetic acid (EDTA) cannot be absorbed by the gastrointestinal tract. Intravenous injection of sodium EDTA causes hypocalcemic hand-foot twitching. It is commonly used for plasma or molecular diagnostics, and calcium chelation. EDTA can be used as an anticoagulant in blood cell count and cell morphology analysis.
• Detergent. EDTA can be used as a labeling buffer component for washing during cell lysis {155}.
• Supplements. As a supplement for synthetic tubal fluid culture 1 (SOFC1) medium for embryo culture.
• Cell sorting analysis. Firstly, single-cell suspension of spleen dendritic cells for magnetic bead cell sorting. Secondly, incubate EpCAM + cells and FITC-labeled antibodies for cell sorting analysis.
References
[1] Oviedo C, Rodríguez J. EDTA: the chelating agent under environmental scrutiny [J]. Quimica Nova, 2003, 26(6): 901-905.
[2] Song Y, Huang Z, Song Y, et al. The application of EDTA in drug delivery systems: doxorubicin liposomes loaded via NH4EDTA gradient [J]. International journal of nanomedicine, 2014, 9: 3611.
[3] Ali A, Sirard M A. Effect of the absence or presence of various protein supplements on further development of bovine oocytes during in vitro maturation [J]. Biology of Reproduction, 2002, 66(4): 901-905.
[4] Schmelzer E, Wauthier E, Reid L M. The phenotypes of pluripotent human hepatic progenitors [J]. Stem cells, 2006, 24(8): 1852-1858.
[5] Menges M, Rößner S, Voigtländer C, et al. Repetitive injections of dendritic cells matured with tumor necrosis factor α induce antigen-specific protection of mice from autoimmunity[J]. The Journal of experimental medicine, 2002, 195(1): 15-22.
[6] McPherson R A, Pincus M R. Henry's clinical diagnosis and management by laboratory methods E-book[M]. Elsevier Health Sciences, 2017.
[7] Yaakobi T, Roth D, Chen Y, et al. Streaming of proteolytic enzyme solutions for wound debridement: a feasibility study [J]. Wounds, 2004, 16(6): 201-205.
- Related articles
- Related Qustion
- EDTA : a chelating agent and anticoagulant Apr 28, 2024
EDTA is a polyprotic acid containing four carboxylic acid groups and two amine groups with lone-pair electrons that chelate calcium and several other metal
- Ethylenediaminetetraacetic acid: Application, toxicity and environmental fate May 9, 2023
Ethylenediaminetetraacetic acid was developed by Franz Munz in Germany during the 1930s as an alternative to citric acid. EDTA is used as a food additive, in herbicides, and pharmaceuticals.
- The principal toxicity of EDTA Oct 18, 2021
Ethylenediaminetetraacetic acid (EDTA) was developed by Franz Munz in Germany during the 1930s as an alternative to citric acid. EDTA is used as a food additive, in herbicides, in pharmaceuticals, and in a variety of consumer products.
Acetyl chloride [75-36-5], C2H3OCl, mol wt 78.50, is a colorless, corrosive, irritating liquid that fumes in air. It has a stifling odor and reacts very rapidly with water, readily hydrolyzing to acetic acid and hydrochloricacid.....
Apr 1,2020Organic Raw MaterialN,N-Diisopropylethylamine is also known as Hunig’s base and abbreviated as DIPEA or DIEA, N,N-Diisopropylethylamine is a sterically hindered amine and an organic compound.....
Jun 29,2020AmidesEthylenediaminetetraacetic acid
60-00-4You may like
Ethylenediaminetetraacetic acid manufacturers
- Ethylene Diamine Tetraacetic Acid
-

- $6.00 / 1kg
- 2025-03-06
- CAS:60-00-4
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 2000KG/Month
- Ethylenediaminetetraacetic acid
-

- $20.00/ kg
- 2025-03-06
- CAS:60-00-4
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 5000kg/Week
- Ethylenediaminetetraacetic acid
-

- $0.00 / 25KG
- 2025-03-05
- CAS:60-00-4
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 50000KG/month