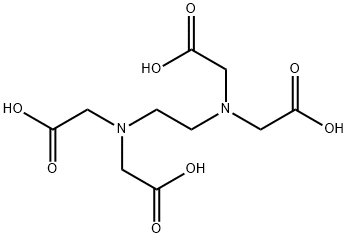
The principal toxicity of EDTA
Oct 18,2021
Ethylenediaminetetraacetic acid (EDTA) was developed by Franz Munz in Germany during the 1930s as an alternative to citric acid. About the same time, Frederick Bersworth synthesized EDTA in the United States using a different technique. He obtained aUS patent for this technique in 1945. EDTA was approved by the US Food and Drug Administration (FDA) as a food additive in 1947. Since the early 1950s, EDTA has been used in chelation treatment for lead poisoning. EDTA is a white, odorless, and crystalline (sugar or sandlike)material.

Environmental Fate
EDTA can be very persistent in water, including wastewatertreatment plants. EDTA is often found in the receiving waters of many industrial areas, thus being classified as one of the major organic pollutants discharged in waters. The available ecotoxicity data for EDTA indicate that these compounds are slow to degrade under typical environmental conditions but are not expected to bioconcentrate. EDTA compounds range from practically nontoxic to moderately toxic on an acute basis, depending on the salt. Algae and invertebrates are among the most sensitive species based on predictive modeling for acute and chronic endpoints for EDTA, depending on the compound. EDTA and its salts also do not appear to be very toxic for terrestrial wild mammals, and adverse effects from reasonably expected agricultural uses are not expected.
The principal toxicity of EDTA relates to the metal chelate, especially in lead poisoning. Lead may be released from the chelate in the kidneys, and then the lead may affect the tubules and glomeruli of the kidneys.
Uses
EDTA is used as a food additive, in herbicides, in pharmaceuticals, and in a variety of consumer products. EDTA is used as a blood preservative by complexing free calcium ion (which promotes blood clotting). EDTA’s ability to bind to lead ions makes it useful as an antidote for lead poisoning. Furthermore, EDTA is often used to treat various cardiovascular diseases.
- Related articles
- Related Qustion
- EDTA : a chelating agent and anticoagulant Apr 28, 2024
EDTA is a polyprotic acid containing four carboxylic acid groups and two amine groups with lone-pair electrons that chelate calcium and several other metal
- Ethylenediaminetetraacetic acid: Application, toxicity and environmental fate May 9, 2023
Ethylenediaminetetraacetic acid was developed by Franz Munz in Germany during the 1930s as an alternative to citric acid. EDTA is used as a food additive, in herbicides, and pharmaceuticals.
- Ethylenediaminetetraacetic acid(EDTA)– Physical Properties and Applications Apr 17, 2020
Edetic acid and EDTA. EDTA is white powder, which is soluble in sodium hydroxide, sodium carbonate and ammonia solution, and 160 parts of boiling water, and slightly soluble in cold water.
2-Acetylaminofluorene (2-AAF) was originally synthesized to be used as a pesticide but due to its profound carcinogenicity it is now purely used in research laboratories for research purposes only. The occupations at greatest risk to acetyl....
Oct 18,2021Organic reagentsEpichlorohydrin is manufactured from allyl chloride and is widely used as a chemical intermediate. A major use is in the synthesis of bisphenol A diglycidyl ether, which is a component of epoxy resins.....
Oct 19,2021Organic SolventsEthylenediaminetetraacetic acid
60-00-4You may like
Ethylenediaminetetraacetic acid manufacturers
- EDTA
-

- $0.00 / 1Kg
- 2025-04-24
- CAS:60-00-4
- Min. Order: 1Kg
- Purity: 99.99%
- Supply Ability: 20 tons
- Ethylenediaminetetraacetic acid/EDTA
-

- $0.00 / 25Kg/Drum
- 2025-04-24
- CAS:60-00-4
- Min. Order: 1KG
- Purity: 99.5%min
- Supply Ability: 20 tons
- Ethylenediaminetetraacetic acid / EDTA FREE ACID
-

- $1.00 / 1kg
- 2025-04-23
- CAS:60-00-4
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 10 mt