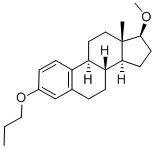
Promestriene: Efficacy, Safety, and Comparisons in Treating Vaginal Atrophy and Genitourinary Syndrome
Apr 24,2025
Promestriene used vaginally to relieve vaginal atrop8hy is a locally effective estrogen that has not shown systemic estrogenic effects. Thus, it could be a first-line option for those who necessitate a minimal or ideally no vaginal absorption, particularly in symptomatic cancer patients. Promestriene safety is further substantiated by its long-lasting and wide clinical experience. Promestriene was first launched in France in 1974. After many millions of boxes of both cream and capsules sold in 34 countries around the world, there is only one published case report of a hematocolpometra in an elderly lady that was believed to be attributable to prolonged and continuous treatment with promestriene cream. Cervical stenosis was caused by atrophy existing before local treatment was started and the intrauterine bleeding could even be unrelated to promestriene use.

Clinical Efficacy of Hormonal and Nonhormonal Agents in the Treatment of Vulvovaginal Atrophy
Symptomatic local treatment of vaginal atrophy (VA) in menopausal women includes hormonal and nonhormonal preparations. Some women may be reluctant to use vaginal estradiol preparations because of the concern for developing breast cancer and endometrial hyperplasia. Therefore, it is necessary to compare the therapeutic effectiveness of alternative vaginal drugs, such as promestriene, an estrogen agonist, and sodium hyaluronate (NaH), a nonhormonal, water-based agent. In this context, preparations containing primarily sodium hyaluronate followed by promestriene may be preferred. As a matter of fact, in the 2013 the North American Menopause Society (NAMS) guideline about the management of symptomatic VVA, it is recommended that first-line treatments should be performed with nonhormonal vaginal lubricants and moisturizers in such risky groups of patients. The NAMS also stated that for symptomatic patients who do not respond to these initial preparations, low dose vaginal estrogens may be an option. This is why we preferred to investigate the effects of promestriene which is a less potent analog of estradiol with minimal systemic absorption property. Promestriene is a well-tolerated, effective vaginal antiatrophic agent in studies evaluating the efficacy and safety of promestriene use.[1]
Del Pup et al. stated that vaginal promestriene is less absorbable than estradiol hemihydrate and to pass into systemic circulation less. Serum estrone sulfate levels were increased in women using vaginal estradiol hemihydrate, whereas no significant change was seen in promestriene users. Del Pub reported that promestriene could be used as the first choice especially in estrogen-associated cancer patients because of minimal absorption and lower systemic hormonal side effects. In our study, we did not find any difference between the groups using vaginal promestriene and vaginal estradiol and HA in terms of serum estradiol levels and endometrial thickness measurements before and after drug use. Despite similar effectiveness with the other two drugs in the reversal of VVA symptoms, it was observed that there was a statistically significant increase in sexual frequency activity in the promestrien group. The average age of women in the estradiol hemihydrate group, which we included in the study, is higher than the HA and promestriene groups. This finding may pose a bias in the evaluation of our study data. To eliminate the negative effect of the age difference between the study groups on the evaluation of the obtained data, the age-adjusted P values (P3) were calculated for the variables with normal distribution and were added to the last column of tables.
In Del Pup et al.'s promestriene review, the duration of drug administration in different studies varies between 1 month and 3 months. For the application of promestriene, it is recommended to apply once a day for the first 20–30 days, then every other day. For vaginal HA, it is recommended to apply one ovule a day for the first 20–30 days, and then one every other day. In conclusion, for the treatment of atrophic vaginitis, in addition to the local estradiol hemihydrate, the use of nonhormonal, water-based vaginal preparations such as sodium hyaluronate or an estrogen agonist with very low or negligible systemic absorption and effective local estrogenic action such as promestriene will provide additional the therapeutic interventions.
Promestriene: is it safe even in cancer patients?
Promestriene have a pharmacodynamic peculiarity because of the presence of 3-propyl and 17b-methyl ether groups. In relation to 17-beta estradiol, this change confers to a promestriene molecule (i) protection against cytochrome P450 (CYP)-based degradation and (ii) it leads to a low plasma concentration, because of less penetration into basal membrane. The metabolism of promestriene is hepatic through CYP3A4 and CYP1A2 enzymes; estradiol is reversibly converted into estrone and estriol. Topic estradiol also undergoes enterohepatic recirculation by conjugation in the liver, followed by excretion of sulfate and glucuronide conjugates into the bile, and then hydrolysis in the intestine and estrogen reabsorption. Sulfate conjugates are the primary form found in postmenopausal women. Estriol and their glucuronide and sulfate conjugates are also excreted in the urine.[2]
Furthermore, the absence of a systemic effect of promestriene has been confirmed recently even with the accurate and sensitive mass spectrometry. The circulating estrogenic pool, evaluated by measuring the variations in the plasma levels of estrone sulfate (E1S), did not change after 1 month of promestriene treatment even in cancer patients with vaginal atrophy .E1S was measured before and 1 month after vaginal promestriene treatment (10 mg/day) in 17 patients with severe vaginal dryness and dyspareunia referred to the gynecological endocrine–oncological service of the Gynecological Oncology Department of the National Cancer Institute of Aviano, Pordenone, Italy. Patients were informed of the aim of the study and of the benefits and risks of promestriene. Two patients were withdrawn during the study: one started a therapy with letrozole and the other died, unrelated to the treatment studied. Of the eligible patients, six had cervical cancer, four had endometrial cancer, three had ovarian cancer, and two had vulvar cancer. Patients’ median age was 48 years (range 26–66). Moreover, to improve its safety, a very low dose of vaginal promestriene could be used at the beginning of treatment, starting with half or less of the usual dose, and then gradually increased till the minimum effective dose, to reduce its already minimal vaginal absorption.
Effects of oxytocin versus promestriene on genitourinary syndrome
The histological analysis of the vaginal wall samples performed before the interventions showed no significant differences in epithelial thickness between the groups (p > 0.05). The comparison between the groups after the treatments showed that both the oxytocin group and the promestriene group had an increase in the vaginal epithelium thickness (p < 0.05). Therefore, both interventions proved to be effective in increasing the thickness of the vaginal epithelium (promestriene p < 0.001 and oxytocin p = 0.017). However, when the medications were compared, the promestriene group showed better efficacy than the oxytocin group (p = 0.036). A pilot study was carried out to initially establish the efficacy of oxytocin in relieving symptoms related to postmenopausal urogenital atrophy and to compare it with the already established effects of promestriene. It is envisaged, in future research, to carry out a study of the non-inferiority of oxytocin in relation to promestriene in women with genitourinary menopause syndrome.[3]
After 90 days of vaginal application of oxytocin and promestriene, the authors concluded that oxytocin and promestriene were effective, with no significant difference, in improving the FSFI scale scores of lubrications, satisfaction, and pain; improved the alterations in the vaginal mucosa seen on clinical examination, but without statistical significance and were effective in increasing the thickness of the vaginal epithelium, with promestriene being superior.
References
[1]Ilhan G, Aslan MM, Cevrioglu AS, Y?ld?r?m M, Erkorkmaz U. Clinical Efficacy of Hormonal and Nonhormonal Agents in the Treatment of Vulvovaginal Atrophy. J Menopausal Med. 2021 Apr;27(1):15-23.
[2]Del Pup, Lino et al. “Promestriene, a specific topic estrogen. Review of 40 years of vaginal atrophy treatment: is it safe even in cancer patients?.” Anti-cancer drugs vol. 24,10 (2013): 989-98.
[3]Santos LPA, Bonduki CE, Dardes RCM, Heinke T, Patriarca MT. Effects of oxytocin versus promestriene on genitourinary syndrome: a pilot, prospective, randomized, double-blind study. Clinics (Sao Paulo). 2022 Oct 1;77:100116.
- Related articles
- Related Qustion
Perfluorodecalin serves as an in situ extraction system to enhance the production of alkannin/shikonin in Arnebia euchroma cell suspension cultures.....
Apr 24,2025Chemical MaterialsThe derivatives of per?uorooctyl iodide (PFOI) are widely used, so PFOI as an intermediate in chemical industry, has a higher commercial value.....
Apr 24,2025Organic Synthesis IntermediatePromestriene
39219-28-8You may like
- Promestriene
-

- $0.00 / 1kg
- 2025-04-24
- CAS:39219-28-8
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 2000
- Promestriene
-

- $0.00 / 1kg
- 2025-04-24
- CAS:39219-28-8
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 600kg
- Promestriene
-
- $1.10 / 1g
- 2025-04-17
- CAS:39219-28-8
- Min. Order: 1g
- Purity: 99.0% min
- Supply Ability: 100 tons min