Glycidyl methacrylate: versatile applications in biomedical field
Jun 30,2023
General Description
Glycidyl methacrylate (GMA) is a highly reactive monomer with an epoxy group and a methacrylate functional group. Its reactivity allows for polymerization using various techniques. The epoxy group also enables further modifications and the introduction of specific functionalities into resulting polymers. Glycidyl methacrylate's crosslinking ability improves mechanical strength and thermal stability in materials. Its excellent adhesive properties make it suitable for strong bonding and adhesion to different surfaces. Glycidyl methacrylate's hydrophobicity enables compatibility with hydrophobic polymers, enhancing overall performance. Moreover, glycidyl methacrylate demonstrates good biocompatibility, making it valuable for biomedical applications. Exciting developments include the use of glycidyl methacrylate as a healing agent in waterborne polyurethanes and as a crosslinking agent in cardiovascular biomaterials. Additionally, glycidyl methacrylate is utilized in solid-phase extraction for selective enrichment and analysis of phosphorylated proteins and as a compatibilizer in designing nanocomposites. These discoveries highlight the broad range of potential applications for glycidyl methacrylate and its significant contributions to various industries.

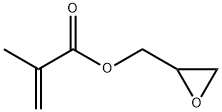
Figure 1. Glycidyl methacrylate
![]() Applications in biomedical field
Applications in biomedical field
Healing agents of waterborne polyurethanes
Glycidyl methacrylate demonstrates its potential as a healing agent in waterborne polyurethanes (WPUs). By incorporating glycidyl methacrylate-based copolymers, such as the hydrophobic P(BA-co-GMAy) and amphiphilic P(PEGMA-co-GMAy), into WPUs, homogenous composite materials are formed. The addition of P(BA-co-GMAy) results in amorphous materials, while the addition of P(PEGMA-co-GMAy) leads to hybrid composites that can range from amorphous to semi-crystalline based on the copolymer or blend composition. These copolymers demonstrate promising healing efficiency when triggered by water. 1
Cardiovascular biomaterial
In cardiovascular biomaterials, glycidyl methacrylate demonstrates promising characteristics when used as a crosslinking agent. A study focused on utilizing glycidyl methacrylate-based radical polymerization to crosslink decellularized swim bladders as an alternative material for artificial biological valves. By reacting the amino and carboxyl groups in the swim bladder with epoxy groups on glycidyl methacrylate, carbon-carbon double bonds were introduced, effectively creating glycidyl methacrylate-crosslinked swim bladders (glycidyl methacrylate-SBs). The results indicate several notable advantages of GMA-SBs compared to the commonly used glutaraldehyde-crosslinked swim bladders (GLUT-SBs). Glycidyl methacrylate-SBs showed a 35% decrease in platelet adhesion, highlighting their reduced thrombogenicity. Additionally, the superior anticoagulant property of glycidyl methacrylate-SBs was further confirmed through the ex vivo arteriovenous shunt assay. Furthermore, glycidyl methacrylate-SBs exhibited effective inhibition of calcification when implanted subcutaneously in rats, emphasizing their improved resistance to calcification compared to GLUT-SBs. These findings establish glycidyl methacrylate-SBs as having enhanced antithrombotic and anticalcification properties, making them a promising candidate for cardiovascular biomaterial applications. 2
Material for solid-phase extraction
Glycidyl methacrylate plays a key role in phosphoproteomics for selective enrichment and analysis of phosphorylated proteins. It is used as a material for solid-phase extraction (SPE) when derivatized with imino-diacetic acid (IDA) and bound to Fe(III). The resulting GMA-IDA-Fe(III) particles form irregular agglomerates as observed in electron microscopy. The metal capacity of Fe(III) on GMA-IDA-Fe(III) is determined to be 25.4 micromol/mL using inductively coupled plasma (ICP) analysis. GMA-IDA-Fe(III) is combined with Silica C18 in a cartridge to achieve preconcentration and desalting in a single step. Methyl esterification (ME) is performed to prevent binding of nonphosphorylated peptides to the IMAC function. The SPE method utilizing GMA-IDA-Fe(III) demonstrates a 92% recovery rate for standard phosphopeptides. This system is suitable for selective enrichment and analysis of phosphorylated proteins, demonstrated by successful enrichment of beta-casein-derived phosphopeptides and analysis of in vitro phosphorylated proteins. 3
PLA-based nanocomposites
The use of poly(glycidyl methacrylate) (PGMA) grafted onto cellulose nanocrystals (CNCs) via surface-initiated atom transfer radical polymerization facilitates the compatibility of hydrophilic CNCs with hydrophobic polymers in the design of nanocomposites. This approach introduces a reactive epoxy-containing monomer that serves as a platform for further modifications or crosslinking. Characterization techniques such as XRD, DLS, FTIR, XPS, and elemental analysis confirm the successful grafting of approximately 40% of the polymer onto the CNC surface within just 1 hour of polymerization. The resulting nanocomposites, incorporating 10 wt% of CNC-PGMA-Br as nanofillers in a poly(lactic acid) matrix, demonstrate improved compatibilization, as observed through scanning electron microscopy. 4
Reference
1. Tzoumani I, Soto Beobide A, Iatridi Z, Voyiatzis GA, Bokias G, Kallitsis JK. Glycidyl Methacrylate-Based Copolymers as Healing Agents of Waterborne Polyurethanes. Int J Mol Sci, 2022, 23(15):8118.
2. Li M, Zheng C, Wu B, et al. Glycidyl methacrylate-crosslinked fish swim bladder as a novel cardiovascular biomaterial with improved antithrombotic and anticalcification properties. J Biomater App, 2022, 36(7):1188-1200.
3. Aprilita NH, Huck CW, Bakry R, et al. Poly(glycidyl methacrylate/divinylbenzene)-IDA-FeIII in phosphoproteomics. J Proteome Res, 2005, 4(6):2312-2319.
4. Le Gars M, Bras J, Salmi-Mani H, et al. Polymerization of glycidyl methacrylate from the surface of cellulose nanocrystals for the elaboration of PLA-based nanocomposites. Carbohydr Polym, 2020, 234:115899.
- Related articles
- Related Qustion
- Glycidyl Methacrylate: A Key Compound in Self-Healing Technologies and Enhanced Cardiovascular Biomaterials Aug 29, 2024
Glycidyl methacrylate enhances self-healing materials and cardiovascular biomaterials, improving biocompatibility, antithrombotic properties, and allowing effective self-repair.
- The toxicity of Glycidyl methacrylate Jun 7, 2024
Glycidyl Methacrylate (GMA) is a clear, colourless liquid with a strong ester and fruity odour.
- The applications of Glycidyl methacrylate Sep 25, 2019
Glycidyl methacrylate (GMA) is an ester of methacrylic acid and glycidol. It is a common monomer used in the production of epoxy resins. GMA is also a high purity, dual functionality monomer ideally suited for coating and resin applications
BHA is a widely used antioxidant in food products, preventing oxidative reactions and maintaining the quality of various foods during processing and storage. However, it has some potential hazards.....
Jun 29,2023APITrimethoxymethane is a valuable reagent in organic synthesis and playing a crucial role in organic reactions.....
Jul 3,2023APIGlycidyl methacrylate
106-91-2You may like
Glycidyl methacrylate manufacturers
- Glycidyl methacrylate
-

- $30.00 / 1kg
- 2024-10-18
- CAS:106-91-2
- Min. Order: 1kg
- Purity: 98%
- Supply Ability: 2000kg
- Glycidyl methacrylate
-

- $99.00/ kg
- 2024-10-18
- CAS:106-91-2
- Min. Order: 0.0010000000474974513kg
- Purity: 99%
- Supply Ability: 5000
- Glycidyl methacrylate
-

- $0.00 / 1KG
- 2024-10-17
- CAS:106-91-2
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 500000kg