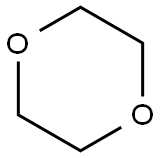
How to synthesis 1,4-Dioxane?
Jul 2,2024
Introduction
1,4-Dioxane, a cyclic ether first reported synthesized in 1863, poses a cancer risk when released into the air and people breathe it, but the chemical doesn't stick around in outdoor air. According to the EPA, it degrades quickly in the atmosphere, with a half-life of less than 5 h. The compound reacts with photochemically produced hydroxyl radicals to form breakdown products such as aldehydes and ketones. As the US Clean Air Act requires, the EPA regulates 1,4-dioxane as part of a family of substances classified as hazardous air pollutants. But in water, it dissolves completely, even at high concentrations. It also does not evaporate readily. These properties make 1,4-dioxane difficult to remove from water.
The compound 1,4-dioxane is a trace contaminant in some cosmetic products. It is not used as an ingredient in cosmetics but may be present in extremely small amounts in some cosmetics. 1,4-dioxane forms as a byproduct during the manufacturing process of certain cosmetic ingredients. These ingredients include certain detergents, foaming agents, emulsifiers and solvents identifiable by the prefix, word, or syllables "PEG," "Polyethylene," "Polyethylene glycol," "Polyoxyethylene," "-eth-," or "-oxynol-."
Uses
However, 1,4-Dioxane is crucial in our life. Besides being used as an aprotic polar solvent with a fragrance, it has many uses beyond its key role as a stabilizer for many chemicals. It is used directly in several industrial and commercial processes. It is found in a wide range of consumer products, such as in medicine, perfume, brightener, aseptic, and synthetic leather, as well as in special fine chemicals production, etc. 1,4-dioxane also occurs as a byproduct in the production of certain surfactants; synthetic textiles, plastics, and resins. 1,4-Dioxane can also be used in the new energy field. Ding et al. found that 1,4-dioxane could be used in passivating lithium electrodes. Choi et al. reported that a 1,4-dioxane diol derivative compound was applied as a secondary battery electrolyte additive used in an electrolyte solution for a lithium secondary battery. Yamaji et al. discovered that 1,4-dioxane could replace lithium ion secondary battery electrolyte[1].
Synthesis
There are a variety of methods known for the continuous preparation of 1,4-dioxane. The most widely employed commercial methods for the continuous preparation of 1,4-dioxane consists of dehydrating ethylene glycol, a polyethene glycol, a mixture of ethylene glycol and one or more polyethene glycol(s) or a mixture of polyethene glycols in the presence of a sulfuric acid catalyst. The dehydration reaction is conducted at an elevated temperature, usually above 170° C. and at atmospheric pressure. The reaction product, which consists of 1, 4 dioxane, water and byproducts, is continuously removed from the reaction mixture as an overhead product, condensed, and the 1,4-dioxane subsequently separated from the water and byproducts. As the 1,4-dioxane is removed from the reactor and the sulfuric acid is deactivated, additional amounts of glycol(s) and sulfuric acid are added to the reactor. Unfortunately, severe charring and the formation of tars are inherent in the sulfuric acid-catalyzed method for preparing 1,4-dioxane, which results in losses in product yield. In addition, foaming in the reactor causes the tars to be carried over into the product, thereby causing discolouration of the reaction product. The formation of tar and its subsequent carry-over into the product require the vessels and equipment used in the process to be cleaned on a frequent basis[2].
In industrial processes, there are many side reactions in the production of 1,4-dioxane from ethylene glycol dehydration with concentrated sulfuric acid, and the purity of 1,4-dioxane is very low. Moreover, corrosion by sulfuric acid leads to high requirements for manufacturing equipment. Serious environmental pollution should also be considered because of the high volumes of wastewater and industrial salt from neutralising sodium hydroxide with sulfuric acid.
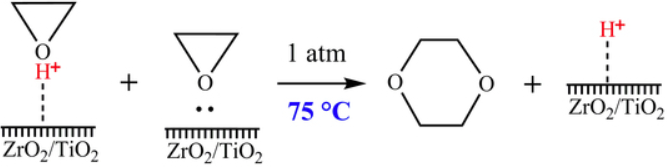
Wang et al. report a route to produce 1,4-dioxane from oxirane by using ZrO2/TiO2 as a catalyst[1]. The composite oxide ZrO2/TiO2 was prepared using a coprecipitation method, and the catalytic performance was tested by synthesising 1,4-dioxane from oxirane in a pipe reactor. The reaction mechanism was analyzed according to the distribution of the product. The test of catalytic performance showed 100.0% conversion of oxirane and 86.2% selectivity of 1,4-dioxane at the optimal operation conditions: atmospheric pressure, reaction temperature 75 °C and gaseous hourly space velocity of 1200.0 h−1. The catalyst exhibits good catalytic performance stability after continuous use for 720 h.
References
[1] Yinan Wang. “Selective Synthesis of 1,4-Dioxane from Oxirane Dimerization over ZrO2/TiO2 Catalyst at Low Temperature.” Catalysts (2022).
[2] US4764626A - Method for producing 1,4-dioxane - Google Patents. https://patents.google.com/patent/US4764626A/en.
- Related articles
- Related Qustion
- Uses of 1,4-Dioxane Jan 19, 2022
1,4-Dioxane was first identified in 1863 and became available for commercial use in the 1930s as a solvent in cellulose acetate and plastics manufacturing. 1,4-Dioxane was used as a stabilizer for chlorinated organic solvents, beginning in
- What is the application of 1,4-Dioxane? Oct 13, 2021
1,4-Dioxane was used as a stabilizer for chlorinated organic solvents, beginning in the 1950s. Groundwater contamination has been an issue of concern near hazardous waste sites contaminated with chlorinated solvents.
- 1,4-Dioxane-Hazard and Toxicity Sep 5, 2019
1,4-dioxane is a clear liquid with ether-like odour. It is highly flammable and forms explosive peroxides in storage (rate of formation increased by heating, evaporation, or exposure to light). 1,4-Dioxane is incompatible with oxidising age
Oxybenzone is harmful to marine wildlife species. Oxybenzone can directly enter the ocean when vacationers, swimmers, surfers, and the like use toxic sunscreen on their bodies and get in the water.....
Jul 2,2024Organic Chemistry(1R)-(-)-10-Camphorsulfonic acid is a non-alkyl acid containing an oxidising group that may be involved in hydrogen bonding and is mainly used to separate optically active isomers.....
Jul 2,2024API1,4-Dioxane
123-91-1You may like
- Phenothiazine and its Derivatives
Aug 5, 2024
- The brief introduction of Isoamyl nitrite
Aug 5, 2024
- Indole Synthesis: Methods and Mechanisms
Aug 2, 2024
- 1,4-Dioxane
-

- $2740.00 / 1ton
- 2024-08-02
- CAS:123-91-1
- Min. Order: 1ton
- Purity: 99%
- Supply Ability: 5000ton
- 1, 4-Dioxane
-

- $10.70 / 1KG
- 2024-07-25
- CAS:123-91-1
- Min. Order: 10KG
- Purity: 99%
- Supply Ability: 10000kg
- 1,4-Dioxane
-

- $50.00 / 1KG
- 2023-12-23
- CAS:123-91-1
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: g-kg-tons, free sample is available