Josamycin:Uses,Pharmacodynamics,Toxicity,Preparation
Dec 27,2022
General description
The

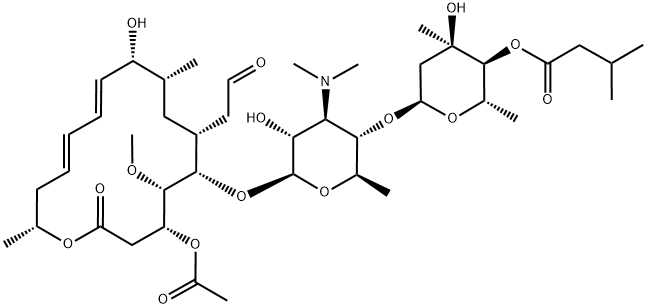
Figure 1 The molecular formula of Josamycin
Uses
Josamycin is sensitive to Ureaplasma urealyticum. At present, it has been used as one of the priority drugs to treat urinary tract infection and guide clinical drug selection. Josamycin is used to treat oral diseases and has also achieved significant clinical effects [3]. Josamycin has strong antibacterial activity against gram-positive aerobic bacteria and various anaerobic bacteria that cause acute oral infection. After clinical trial in many hospitals, it was found that Josamycin was effective in treating respiratory tract infection, and had no damage to liver and kidney functions, and no obvious gastrointestinal reaction [4]. Josamycin can be made into dry syrup to increase the compliance of children. Its fatty compound, Josamycin propionate, has a smaller gastrointestinal reaction. Aromatic granules can easily solve the problem of children taking medicine. Relatively speaking, Josamycin has better drug compliance and smaller gastrointestinal reaction, which is more suitable for children [4].The overall antibacterial activity of Josamycin is similar to erythromycin, and it is effective against mycoplasma, gram-positive (staphylococcus, gonococcus, diplococcus), bacilli and some gram-negative bacteria [5]. However, it has the advantage of no toxicity on blood, kidney and liver, meanwhile, no side effects even at high doses, and no inhibition on cytochrome P450 enzyme activity [5]. Because Josamycin inhibits the activity of peptidyltransferase through peptide extension, it also has activity against some resistant bacteria. It is also reported that leucomycin analogues have high antibacterial and anticancer activities. This type of antibiotic can be a candidate for antibacterial and anticancer drugs. Josamycin and its derivatives have relatively good gastric tolerance, low tendency to produce allergy and low induced resistance [6].
Pharmacodynamics
Oral fasting administration of Josamycin 1g, the average peak time (Tmax) was 1.03 ± 0.41 h, and the average peak blood concentration (Cmax) was 3.22 ± 2.21 mg/L. At 6 h, the blood concentration was 0.39 ± 0.42 mg/L. The in vivo process of Josamycin conforms to the one-compartment model. The absorption of Josamycin was fast, the average absorption half-life was 0.3 ± 0.3 h, and the elimination half-life was 1.7 ± 0.42 h. The area under the drug time curve (AUC) was 6.66 ± 4.33 hmg/L, and the relative bioavailability was 116%. The renal clearance rate was 76.2 ± 53 mL/min. After oral administration of 1 g of Josamycin, 1.12 ± 2.21% of the average dose was excluded from the urine 24 hours later. The urine peak concentration appeared within 3 h after administration, with an average of 51.4 ± 61.3mg/L[7].
Mechanism of action
The mechanism of action of Josamycin is different from that of erythromycin. Josamycin has dual growth inhibition in living cells: (1) It blocks the activity of peptidyltransferase on ribosomes. (2) By inducing the shedding of a large amount of dipeptide tRNA in ribosomes, the tRNA used to synthesize proteins in cells is reduced [8]. In terms of anti-inflammatory activity, the inhibition of p38MAPK signal pathway mediated by Josamycin leads to its effect on neutrophils, other immune cells and anti influenza effect in vivo. In recent studies on the target of Josamycin, it was found that mitochondrial ribosomes are the direct binding target of Josamycin, and this interaction is also the reason for its effect on mitochondrial function [8].
Toxicity
The most common adverse effects include skin rash and gastrointestinal reactions including nausea, vomiting, anorexia, abdominal distension, diarrhea. There were occasional drug eruptions, and no liver damage and other adverse reactions were found.
Preparation
The biosynthetic lactone ring of Josamycin is formed by condensation of 5 acetic acid units, 1 propionic acid unit, 1 butyric acid unit and 1 glycolic acid unit through the polyketone pathway similar to that of fatty acid synthesis. The basic process is that after the activation of low-grade fatty acids, the carbon chain is continuously extended by polyketide condensase mediated by acyl carrier protein in the form of malonyl, propionyl or methyl malonyl, and finally the carbon chain is cyclized by thioesterase to form polyketolactone. The extension of carbon chain can be accompanied by reduction, dehydration and other reactions, leading to polyketone. The main disadvantage of Josamycin is that it is easy to be enzymatically hydrolyzed in the body and inactivated by opening the lactone ring or removing the phthalic group [9]. However, this disadvantage can be overcome by chemical modification of its structure. At present, there are few effective semi synthetic new varieties modified with Josamycin, and Josamycin propionate is widely used. Its structural formula is as follows:

Figure 2 The molecular formula of Josamycin propanoate
[1] Reese R E, Betts R F, Goedde L W, et al. Invitro Susceptibility of Common Clinical Anaerobic and Aerobic Isolates against Josamycin. Antimicrobial Agents and Chemotherapy. 1976, 10(2): 253-257.
[2] Privitera G, Bonino S, Delmastro S. Clinical Multicenter Trial with Josamycin Propionate in Pediatric-Patients. International Journal of Clinical Pharmacology Research. 1984, 4(3): 201-207.
[3] Karamova A E, Polyakov A V, Komarova N V. Detection of Mutant Mycoplasma Hominis Strains Resistant to-16-Membered Macrolide Antibiotic Josamycin in Clinical Samples. Bulletin of Experimental Biology and Medicine. 2004, 137(5): 483-484.
[4] Debernardi C, Bondone B, Rolle G, et al. The Use of Josamycin in Dental Pathology. Dental Cadmos. 1989, 57(1): 74-77.
[5] Chalker V J, Sharratt M G, Rees C L, et al. Tetracycline Resistance Mediated by Tet(M) Has Variable Integrative Conjugative Element Composition in Mycoplasma Hominis Strains Isolated in the United Kingdom from 2005 to 2015. Antimicrobial Agents and Chemotherapy. 2021, 65(4):e02513-20.
[6] Song X, Bao D, Lyu J, et al. Analysis on Status and Drug Sensitivity of 1257 Suspected Female Genital Tract Mycoplasma Infection. Chinese Journal of Nosocomiology. 2021, 31(23): 3628-3631 1005-4529(2021)31:23<3628:1lmzns>2.0.Tx;2-8.
[7] Liu Y-M, Shi Y-M, Liu Z-L, et al. A Sensitive Method for Simultaneous Determination of Four Macrolides by Ce with Electrochemiluminescence Detection and Its Applications in Human Urine and Tablets. Electrophoresis. 2010, 31(2): 364-370.
[8] Zeng T, Wu Y, Yang Z, et al. Clinical and Microbiological Characterization of Bloodstream Infections Caused by Mycoplasma Hominis: An Overlooked Pathogen. Infectious Diseases and Therapy. 2022, 11(3): 1003-1017.
[9] Berthe-Aucejo A, Girard D, Lorrot M, et al. Evaluation of Frequency of Paediatric Oral Liquid Medication Dosing Errors by Caregivers: Amoxicillin and Josamycin. Archives of Disease in Childhood. 2016, 101(4): 359-364.
- Related articles
- Related Qustion
- Josamycin: Pharmacodynamics, Pharmacokinetics and Indications Apr 8, 2024
Josamycin, a macrolide antibiotic, inhibits bacterial protein synthesis and is effective against various infections due to its unique pharmacokinetic profile.
Levomefolate calcium is an organic calcium salt,it has a role as an antidepressant.Levomefolate calcium is the active metabolic form of folates in the body.....
Dec 26,2022APIFormic acid is a colorless liquid with a strong pungent odor. It is used in the dyeing and finishing of textiles and paper, in the treatment of leather, and in the manufacture of many other chemicals.....
Dec 27,2022Organic ChemistryJosamycin
16846-24-5You may like
- 7-Azaindole: Uses and Synthesis
Sep 11, 2024
- Medroxyprogesterone Acetate: A Comprehensive Overview
Sep 11, 2024
- Cyanoacetamide: A Versatile Compoun....
Sep 11, 2024