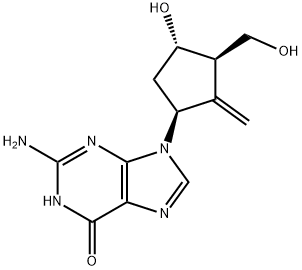
Mechanism of action of Entecavir
Apr 6,2022
Entecavir (BMS-200475) is a guanosine nucleoside analog with selective activity against hepatitis B virus (HBV), as well as some activity toward HIV. It is marketed as Baracludes, and received regulatory approval in the USA in March 2005, in Australia in April 2006, and in Europe in June 2006.
The chemical name for entecavir is 2-amino-1,9-dihydro-9 [(1S,3R, 4S)-4-hydroxy-3-(hydroxymethyl)-2-methylenecyclopentyl]-6H-purin-6- one monohydrate. Its molecular formula is C12H15N5O3·H2O, which corresponds to a molecular weight of 295.3. The chemical structure of entecavir is shown below. It is slightly soluble in water (2.4mg/ml) and the pH of the saturated solution in water is 7.9 at 25°C.
Uses
Entecavir is a useful drug for treatment of HBV infection, for both patients with and without detectable HBeAg. Entecavir efficacy in comparison with lamivudine was studied in three large phase III trials. All studies were randomized, double-blinded, and placebo controlled, and included patients W16 years of age with elevated ALT (1.3 to 10 times the upper limit of normal) and with compensated liver disease; patients with HIV, hepatitis C, or hepatitis D virus coinfection were excluded. Nucleoside-naive patients were given 0.5 mg entecavir daily, whereas lamivudine-refractory patients were treated with 1mg daily.
Mechanism of action
Like other nucleoside and nucleotide antivirals, entecavir is a prodrug which must be phosphorylated to entecavir triphosphate, the active form of the drug, intracellularly. Once entecavir enters a cell, triphosphorylation occurs rapidly and efficiently, mediated by cellular nucleoside kinases. By competing directly in a dose-dependent manner with deoxyguanosine triphosphate (dGTP), entecavir triphosphate potently inhibits each of the three distinct activities of the HBV viral polymerase: priming, reverse transcription of first-strand DNA synthesis, and the DNA-dependent DNA polymerase activity responsible for second-strand DNA synthesis. entecavir triphosphate is a competitive inhibitor of the HBV reverse transcriptase, and this enzyme in fact displays a higher affinity for entecavir triphosphate (Ki of 1.3–2.6 nM) than it does for its natural substrate, dGTP (Km of 13.3–20 nM).
Measurement of the extent to which entecavir triphosphate can substitute for the natural substrate dGTP in an oligonucleotide primer extension assay showed that extension of the primer by one nucleotide (+1) was 12–26 times less efficient with entecavir substituting for dGTP, and a three-nucleotide (+3) extension of entecavir-terminated primers was 16–40 times less efficient than those terminating with dGP. Therefore, entecavir acts as a nonobligate chain terminator, usually two or three nucleotides downstream from its incorporation into DNA.
Mycophenolic acid and ribavirin markedly enhance the antiviral effect of entecavir against wild-type HBV and the eM204V lamivudine-resistant HBV in vitro. The mechanism relates to the fact that both compounds inhibit IMP dehydrogenase, causing depletion of intracellular dGTP pools, which increases the ability of entecavir triphosphate to compete with dGTP and thus inhibit the HBV polymerase.
Bioavailability
Entecavir is well absorbed orally, with W70% of the drug reaching plasma. Although co-administration with food decreases absorption by 20%, this is not considered to be clinically significant. Maximum plasma concentrations of entecavir are reached in less than 2 hours. Entecavir is not bound significantly to plasma proteins (B13%) and uniformly distributes between plasma and red blood cells. Entecavir has an elimination half-life (t1/2) of approximately 130 hours, with an effective half-life for absorption of 24 hours; steady state is achieved after repeated daily doses for 9–10 days. At steady state, entecavir dosing of 0.5mg daily resulted in mean maximum concentrations (Cmax) of 4.23 ng/ml and 24-hour exposure (AUC24h) of 14.7 ng/ml h. After repeated dosing of 1mg daily, mean Cmax was 8.26 ng/ml and AUC24 h was 26.4 ng/ml h (i.e. pharmacokinetics were dose-proportional). Inter-subject variation in these variables was less than 20% in phase I studies.
Drug interactions
Entecavir does not appear to be a substrate, inhibitor, or inducer of the CYP450 enzyme system. In vivo studies indicate that there are no significant pharmacokinetic interactions with lamivudine, adefovir, or tenofovir. In vitro studies suggest that there are no pharmacodynamic interactions with stavudine, didanosine, abacavir, zidovudine, lamivudine, or tenofovir that would affect their activity toward HIV or HBV.
Side-effects
The safety profile of entecavir appears similar to that of lamivudine in comparative trials. Doses of up to 20 mg (20–40 times the recommended dose) for 14 days were administered in phase I studies without any significant clinical or laboratory adverse events. The most common side-effects reported in clinical trials (headache, abdominal pain, diarrhea, fatigue, arthralgia, dizziness, nausea, dyspepsia, increased liver enzymes, increased blood amylase, back pain, and myalgia) were generally mild and were considered to be unrelated to the study drug. The overall rate of serious adverse events was 7% in treatment-naive patients and 10% in lamivudine-refractory patients. Treatment was discontinued because of an adverse event (clinical or laboratory) in only 1% of entecavirtreated patients, compared with 4% of lamivudine-treated patients. Those adverse events possibly, probably, or definitely related to medication are shown in Table 247.4. Proportions of adverse effects in entecavir-treated patients were less than or equal to those seen in lamivudine-treated patients.
Serious adverse events and rate of discontinuation were very low in all entecavir clinical trials. In all randomized comparative clinical trials, mortality rates in entecavir-treated and lamivudine-treated patients were equivalent. One death was possibly due to cessation of entecavir treatment and subsequent hepatic decompensation. Entecavir does not appear to be associated with significant neurologic toxicity or lactic acidosis. Entecavir has no activity against gamma DNA polymerase of mitochondria and only low levels of activity against other cellular DNA polymerases.
- Related articles
- Related Qustion
- How long does Entecavir take to treat patients with chronic hepatitis B (CHB)? Jun 5, 2024
Months ~ years or permanently. Entecavir (ETV) is an oral prescription antiviral medication used to treat liver infections caused by the hepatitis B virus.
- Entecavir: Highly Effective Antiviral Drug for Hepatitis B Treatment Feb 6, 2024
Entecavir is an effective antiviral for chronic hepatitis B, inhibiting viral replication. Side effects are mild, and resistance can occur in non-naive patients.
- The applications and side effects of Entecavir Nov 6, 2023
Entecavir is an analog of 2′-deoxyguanosine. This compound is approved for the treatment of hepatitis B infection with compensated or decompensated liver disease in adults.
Elvitegravir (brand name Vitekta) is a drug used as part of antiretroviral therapy (ART). The FDA approved elvitegravir in 2012 as an antiretroviral drug (ARV) for people with HIV infection. Elvitegravir is manufactured by Gilead Sciences.....
Apr 2,2022APIAdefovir dipivoxil is a white to off-white crystalline powder with an intrinsic aqueous solubility in water of 19 mg/ml at pH 2 and 0.4 mg/ml at pH 7.2.....
Apr 6,2022APIEntecavir
142217-69-4You may like
- The Synthesis and Hazards of 1,7-Dimethylxanthine
Feb 27, 2025
- Nonapeptide-1 + EGCG: Reverses skin photoaging
Feb 27, 2025
- Diagnostic value of urinary orotic acid
Feb 27, 2025
- Entecavir
-

- $0.00 / 1kg
- 2025-02-27
- CAS:142217-69-4
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 1000
- Entecavir
-

- $100.00 / 25KG
- 2025-02-27
- CAS:142217-69-4
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 5000kg/week
- Entecavir
-

- $0.00 / 1g
- 2025-02-27
- CAS:142217-69-4
- Min. Order: 1g
- Purity: 99% HPLC
- Supply Ability: 1000kg