Memantine HCl: Pharmacokinetics and Therapeutic Efficacy
Jul 11,2024
General Description
Memantine HCl, a medication utilized for Alzheimer's disease and vascular dementia, showcases favorable pharmacokinetics, including complete absorption with linear plasma concentration relationship and renal elimination. Memantine HCl exhibits high bioavailability, long half-life, and minimal drug interactions. In clinical trials for moderate to severe Alzheimer's, memantine demonstrated a significant slowing of cognitive decline compared to placebo, with long-term benefits seen in an open-label extension study. Patients on memantine required less caregiver time, indicating reduced burden. Overall, memantine HCl shows promise in improving cognitive functions and quality of life in individuals with these conditions.

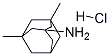
Figure 1. Memantine HCl
Pharmacokinetics
Absorption and Distribution
Memantine HCl is completely absorbed from the gastrointestinal tract with an absolute bioavailability of 100%. Plasma concentrations peak between 3 and 8 hours after oral administration, and the relationship between dose and plasma concentration is linear. Food does not affect the bioavailability of the drug. There is a large interindividual variation in steady state plasma concentrations, with a mean plasma protein binding ratio of 45% and a mean volume of distribution of 10 L/kg.
Biotransformation and Elimination
Approximately 80% of an administered dose of Memantine HCl circulates as unchanged parent drug. Several inactive metabolites have been identified, but cytochrome P450-catalyzed metabolism has not been detected in vitro. The mean terminal elimination half-life of Memantine HCl is 60-100 hours. Memantine HCl is primarily eliminated renally, with 99% of an administered dose recovered in urine. Urinary alkalinization may reduce renal excretion of Memantine HCl. Total body clearance is correlated with creatinine clearance in elderly volunteers.
Metabolism and Drug Interactions
Memantine HCl does not inhibit various cytochrome P450 enzymes in vitro. It also does not have an inhibitory effect on acetylcholinesterase inhibitors. Concomitant administration with donepezil does not affect the pharmacokinetic profile of either agent.
Special Populations
In elderly volunteers with normal or reduced renal function, clearance of Memantine HCl is correlated with creatinine clearance. Renal secretion and reabsorption of Memantine HCl via cationic transport proteins play a significant role in its elimination. Urinary alkalinization may impact the renal excretion of memantine.
Overall, the pharmacokinetics of Memantine HCl are characterized by high bioavailability, linear relationship between dose and plasma concentration, renal elimination, and lack of significant drug interactions. 1
Therapeutic Efficacy
Memantine HCl, a medication used in the treatment of Alzheimer's disease and vascular dementia, has shown promising therapeutic efficacy in clinical trials. This introduction focuses on its effectiveness and impact on patients' cognitive functions and daily living activities.
Clinical Trial in Patients with Moderate to Severe Alzheimer's Disease
In a study conducted in the US, Memantine HCl was evaluated in outpatients with moderate to severe Alzheimer's disease. The patients, aged ≥50 years, demonstrated Alzheimer's disease as per DSM-IV criteria and were at stage 5 or 6 on the Global Deterioration Scale. Key assessments included the Clinician's Interview-Based Impression of Change (CIBIC-Plus), Alzheimer's Disease Cooperative Study Activities of Daily Living Inventory (ADCS-ADL), Severe Impairment Battery (SIB), Mini Mental State Exam (MMSE), and others. Results showed that Memantine HCl treatment significantly slowed the rate of cognitive decline compared to the placebo group.
Long-Term Efficacy and Caregiver Impact
Following the initial 28-week trial, patients who continued Memantine HCl treatment in a 24-week open-label extension study exhibited further improvement in cognitive and functional abilities. Memantine HCl recipients also required significantly less caregiver time compared to those on placebo, indicating a reduced burden on caregivers. Moreover, patients switching from placebo to Memantine HCl experienced a relative improvement in their condition, suggesting a sustained positive impact on disease progression. These findings emphasize the potential of memantine HCl in enhancing the quality of life for individuals with moderate to severe Alzheimer's disease. 2
Reference
1. Greig SL. Memantine ER/Donepezil: A Review in Alzheimer's Disease. CNS Drugs. 2015; 29(11): 963-970.
2. Jarvis B, Figgitt DP. Memantine. Drugs Aging. 2003; 20(6): 465-478.
- Related articles
- Related Qustion
Vitamin D3 regulates gene expression via vitamin D receptor and MARRS, crucial for female fertility by influencing folliculogenesis and oocyte maturation.....
Jul 11,2024APIKetoprofen, chemistry 3-benzoyl by name-Alpha-Methyl toluylic acid, is a non-steroidal anti-inflammatory analgesics, has anti-inflammatory, analgesic, analgesic effects.....
Jul 11,2024APIMemantine HCl
41100-52-1You may like
- Memantine HCl
-

- $1.00 / 1g
- 2024-08-13
- CAS:41100-52-1
- Min. Order: 1g
- Purity: 99%
- Supply Ability: 100kg
- Memantine hydrochloride
-

- $0.00 / 1KG
- 2024-08-07
- CAS:41100-52-1
- Min. Order: 1KG
- Purity: 99
- Supply Ability: 10 tons
- Memantine HCl
-

- $0.00 / 1KG
- 2024-07-25
- CAS:41100-52-1
- Min. Order: 1KG
- Purity: 99.0~101.0%
- Supply Ability: 500KG/month