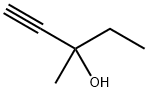
Methylpentynol: Properties, Production process and Uses
Mar 4,2024
Properties of methylpentynol
Methylpentynol is a colorless liquid with low viscosity at room temperature. It has a spicy odor and a burnt smell. The solubility of methylpentynol in water is 12.8% at 25°C. It is miscible with acetone, benzene, carbon tetrachloride, cyclohexanone, ethylene glycol ether, diethylene glycol, ethyl acetate, kerosene, methyl ethyl ketone, ethanolamine, hoof oil, diethyl ether, petroleum ether, soybean oil, dry-cleaning solvent gasoline and the like. Its density is 0.8688 g.cm–3 at 25°C. The melting point is −30.6°C, the boiling point is 121–122°C and the flash point is 38°C. The refractive index is 1.4318 at 20°C. The vapor pressure is 6.5 mmHg at 20°C. It is flammable and toxic.
Production process of methylpentynol
Methylpentynol is prepared by ethynylation of butanone with acetylene:
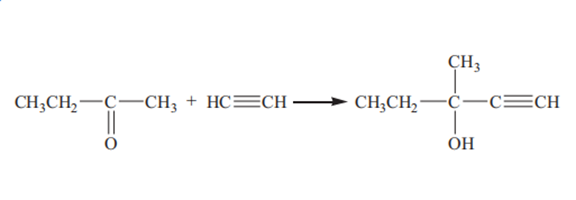
At present, there are three main processes for production of methylpentynol in the industry: ethynylation catalyzed by solid potassium hydroxide, ethynylation catalyzed by liquid ammonia and potassium hydroxide and ethynylation catalyzed by potassium alkoxide.
(1) Ethynylation catalyzed by solid potassium hydroxide
In this process, acetylene is introduced into a solution of an organic solvent (e.g., aromatic hydrocarbon, dichloromethane, diethyl ether and tetrahydrofuran) and butanone in which the powdery solid potassium hydroxide is suspended, and the ethynylation reaction is carried out at near room temperature and under atmospheric pressure. The greatest advantage of this process is that the reaction can be carried out under atmospheric pressure, so that the requirements for equipment are not so harsh, the operation is also very convenient and safe and the equipment investment is low. However, this process also has some shortcomings: First, the powdery potassium hydroxide catalyst will be dissolved in water to form an aqueous potassium hydroxide solution after completion of the reaction, making it difficult to recycle. Since the consumption of solid potassium hydroxide in this method is 3 to 4 times of the consumption of the raw material butanone in terms of moles, the consumption of solid potassium hydroxide is very large and the blockage phenomenon is often caused in the reaction. The treatment of a large amount of alkali liquor is also a difficult problem. Second, due to the use of potassium hydroxide powder, the reaction mixture is particularly viscous and difficult to be fully stirred uniformly in a typical stirred tank. In order to prevent clogging and achieve good mass and heat transfer conditions, a special stirring device or a large amount of solvent is required. Third, the batch operation has a long operation cycle for more than 20 h, and the yield of methylpentynol is as low as less than 70%.
(2) Catalytic ethynylation in liquid ammonia–KOH system
Acetylene is dissolved in liquid ammonia, and butanone and potassium hydroxide catalyst are added. In this process, liquid ammonia not only acts as a solvent but also acts as a cocatalyst. Potassium hydroxide is in the form of an aqueous solution with a mass concentration of 50%, which is used in a very small amount and easy to be recycled. The ethynylation reaction is carried out at a temperature of 30°C and under a pressure of 1.6 MPa for 1 h. The mass ratio of liquid ammonia to butanone is 2.5:1, and the molar ratio of KOH to butanone is 1:359. The yield of methylpentynol is more than 90% based on butanone. Compared with the ethynylation catalyzed by solid potassium hydroxide, the consumption of KOH is greatly reduced. The raw materials and products are all in the liquid phase, and the reaction is homogeneous. It is easy to transfer heat and mass, and easy to control the reaction temperature. It is also easy to achieve continuous production and expand production scale. However, the disadvantage of this process is that the liquid ammonia is highly volatile and requires a pressurization operation, which requires high standard equipment.
(3) Catalytic ethynylation by potassium alkoxide
Potassium alkoxide is used as a catalyst instead of potassium hydroxide. A good catalytic effect can be achieved by using equimolar potassium alkoxide and butanone. The potassium alkoxide can be prepared using potassium hydroxide and an alcohol under atmospheric pressure. Once the synthesis reaction of methylpentynol is completed, the potassium alkoxide can be further decomposed into an alcohol and potassium hydroxide, so that potassium hydroxide can be recycled.
KOH aqueous solution and isobutanol were added to the catalyst preparation kettle connected to a fractional distillation column. Steam was introduced into the jacket of the kettle for heating. During the reaction, the free water and water generated from reaction in the reaction system were removed. Once the reaction was completed, the potassium alkoxide was dispersed in xylene to obtain potassium alkoxide suspension. The suspension was pumped into the ethynylation reaction vessel. First, it was cooled to room temperature with circulating cooling water, and then cooled to near 0°C with chilled brine. Subsequently, acetylene and butanone were introduced into the vessel. The reaction was carried out at 30°C for 3 h. Then water was added in a dropwise manner for hydrolysis. The mixture was allowed to stand for separating into two layers. The oil layer was collected and washed with water, and subjected to fractional distillation for methylpentynol product. The yield of methylpentynol was more than 90% based on butanone.
Catalytic ethynylation by potassium alkoxide has the advantages of operation at atmospheric pressure, less equipment investment, simple process and high yield, and versatility. One set of equipment can produce various alkynol according to production requirements.
Uses of methylpentynol
Methylpentynol was originally used only as a sedative. With the continuous discovery of the properties of Methylpentynol and the continuous improvement of its synthesis technology, the use of Methylpentynol has become more and more widespread. For example, it can be used as a stabilizer for chlorinated solvents, a viscosity reducer and viscosity stabilizer and an intermediate for organic synthesis. In particular, methylpentynol is used as a corrosion inhibitor in the acidification fluid of oil and gas wells.
- Related articles
- Related Qustion
Diethyltoluenediamine (DETDA) is a cycloethylated analog of toluenediamine (TDA) and, like TDA, consists of 2,4 and 2,6-diamine isomers.....
Feb 8,2025APIBaking soda is a white, water-soluble crystalline solid with an alkaline taste, this article will introduce its manufacturing process and uses.....
Mar 4,2024API3-Methyl-1-pentyn-3-ol
77-75-8You may like
3-Methyl-1-pentyn-3-ol manufacturers
- 3-Methyl-1-pentyn-3-ol
-

- $40.00 / 1kg
- 2025-06-20
- CAS:77-75-8
- Min. Order: 1kg
- Purity: 0.99
- Supply Ability: 10 tons
- 3-Methyl-1-pentyn-3-ol
-
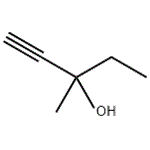
- $1.50 / 1g
- 2023-07-27
- CAS:77-75-8
- Min. Order: 1g
- Purity: 99.0% Min
- Supply Ability: 100 Tons