Pidotimod: uses, Mechanism of action, and Pharmacokinetics
Jun 18,2024
Introduction
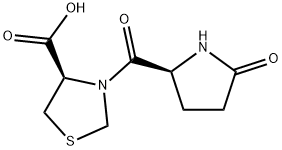
Pidotimod is a synthetic dipeptide molecule (3-l-pyroglutamyl-l-thiazolidine-4carboxilic acid) endowed with immunomodulatory activity that affects both innate and adaptive immune responses. Higher expression of Toll Like Receptors (TLR) 2 and of HLA-DR molecules, induction of DC maturation and release of pro-inflammatory molecules, stimulation of T lymphocyte proliferation and differentiation toward a Th1 phenotype, as well as an increase in the phagocytosis have been demonstrated to be associated with Pidotimod in in vitro studies[1].

Mechanism of action
The immunostimulatory activity of pidotimod is focused on both immune responses – adaptive and innate immunity. It has shown to affect the immune response in multiple ways as enumerated below[2].
Induction of maturation of dendritic cells
Upregulation of HLA-DR and other co-stimulatory molecules (CD83 and CD86) expression
T-cell differentiation toward Th-1 type (via release of pro-inflammatory molecules by stimulating dendritic cells)
Increase in the activity of NK cells
Inhibits thymocyte apoptosis
Promoting phagocytosis
Increase in salivary immunoglobulin (Ig) IgA levels
Upregulation of TLR-7 and TLR-2 signaling pathway in respiratory epithelium (helps to assist in identifying pathogen-associated molecular patterns).
Pharmacokinetics
Pidotimod is rapidly absorbed by the gastrointestinal tract with a bioavailability of 45%. The plasma half life is 4 h with poor metabolism and renal elimination of the unmodified molecule. In vitro studies from both animal and human specimens have documented a good activity on innate and adaptive immune responses and have been confirmed by in vivo studies. These activities have been applied in clinical studies demonstrating the efficacy of pidotimod in reducing the rate of recurrent infections of the upper respiratory and urinary tract in children. The same results were obtained in recurrent respiratory tract infections in adults. More importantly, these effects are more evident in the setting of immune defects such as senescence, Down's syndrome, and cancer.
In therapy
Based on the current evidence, pidotimod should be considered during acute attacks of respiratory infections in children as well as in bacterial exacerbations in adults with CB. For prophylaxis of recurrences in both age groups, pidotimod needs to be continued for at least 2 months after the acute attack. Besides reducing the rate of recurrence, pidotimod reduces the severity of infectious episodes, improves clinical features, results in rapid recovery, reduces the need for antibiotic and other symptomatic treatments, and decreases school absenteeism and visits to pediatric clinics. It improves lung function and airway epithelial clearance, suggesting its potential to lower the rates of asthma in children. Immunostimulation with pidotimod has also paved way for its use in different indications such as CAP, M. pneumonia, acute bronchitis, and HFM disease in children. This indicates that, in major infectious disease where there is immune dysregulation, immunostimulation with pidotimod will be useful. In adults, efficacy in reducing acute exacerbations of CB or COPD with improvement in T-cell function suggests that pidotimod should be considered in the treatment armamentarium of acute exacerbation of bronchitis and COPD. Further consideration in indications such as bronchiectasis suggests its ability to reduce the lower-airway inflammation. Thus, pidotimod can be considered in children and adults with acute and chronic respiratory conditions. Stimulation of innate and adaptive immune responses helps in reducing disease severity in acute phase, and prophylactic use can effectively reduce the recurrences.
References
[1] Francesca Puggioni. “Immunostimulants in respiratory diseases: focus on Pidotimod.” Multidisciplinary Respiratory Medicine 14 (2019): 31.
[2] A. Mahashur. “Pidotimod: In-depth review of current evidence.” Lung India 20 1 (2019): 422–433.
- Related articles
- Related Qustion
Pidotimod
121808-62-6You may like
- What is the Cyclic AMP Pathway?
Oct 12, 2024
- Uridine: Biological function and Biosynthesis
Oct 10, 2024
- How to synthesize Palmitoyl Tripeptide-1?
Oct 9, 2024
- Pidotimod
-

- $65.00/ kg
- 2024-10-12
- CAS:121808-62-6
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 5000kg/week
- Pidotimod
-

- $0.00 / 1Kg/Bag
- 2024-10-11
- CAS:121808-62-6
- Min. Order: 1KG
- Purity: 99%min HPLC;CP2015
- Supply Ability: 500kgs
- pidotimod
-

- $0.00 / 1Kg
- 2024-09-23
- CAS:121808-62-6
- Min. Order: 1Kg
- Purity: 99.9%
- Supply Ability: 200tons