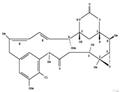
Preparation of Maytansinol
Jan 25,2022
Maytansinol is an expensive starting material and its precursor sources are limited to fermentation processes. The reaction conditions used to produce maytansinoid esters produce roughly equal amounts of the desired stereoisomer, an undesired stereoisomer and unreacted maytansinol.
Bioactivity
Maytansinol inhibits microtubule assembly and induces microtubule disassembly in vitro. Target: Microtubule/Tubulin in vitro: Maytansinol disrupts the mitotic spindle and prevents mitotic exit in Drosophila. Maytansinol reduces the growth and/or survival of HCT116 cells in a dose-dependent manner and that the effect was more severe for p53+/+ than for p53-/- cells at both low and high doses. Maytansinol inhibits the growth of HCT116 human colon cancer cells. Maytansinol induces apoptosis in imaginal discs of wild-type larvae but not p53 mutant larvae. This parallels the finding in human HCT116 cells, in which Maytansinol was more effective when p53 was present, at least at some doses. Maytansinol induces apoptosis in imaginal discs of wild-type larvae but not p53 mutant larvae at 24 hours after exposure to drug.
Preparation of Maytansinol from D-DM1-SMe
A 15 mL septum-capped vial, equipped with a magnetic stirrring bar, was charged with lithium aluminum hydride (1M in THF, 0.52 mL, 5.19×10−4 mole, 4.2 equiv, Aldrich Chemical Co., St. Louis, Mo.) and the solution cooled to −5° C. A solution of N-methyl-N-(3-methyldithiopropanoyl)-D-alanylmaytansine (100 mg, 1.28×10−4 mole, 1 equiv, ChemSyn Laboratories, Lenexa, Kans.) in anhydrous THF (5 mL, Aldrich Chemical Co.) was added at such a rate that the temperature did not exceed 8° C. The reaction mixture was cooled back to −5° C. and the progress of the reaction was monitored by high performance liquid chromotography (HPLC). After 2 h the reaction was complete, the HPLC assay showing maytansinol as the major product, 95.9% by peak area. The product matched the retention times of maytansinol in two different HPLC systems and had the desired molecular mass by LC/MS. The reaction can be worked up by known standard techniques. See Reagents for Organic Synthesis, Volume 1, Feiser and Feiser, pp 583-584, 1967.
- Related articles
- Related Qustion
Benzo[c]furan, also known as isobenzofuran, is a planar unique class of o-quinonoidal bicyclic monooxygen heterocycles with a 10π electron ring system in which the benzene ring is annulated at 3,4-positions of the π-excessive furan ring. T....
Jan 25,2022Organic reagentsDinoprost Tromethamine (Dinolytic, PGF2-alpha tham, Zinoprost, Prostin F2 alpha, Dinoprost Trometamol) is a synthetic analogue of the naturally occurring prostaglandin F2 alpha, which stimulates myometrial activity, relaxes the cervix.....
Jan 25,2022Hormones and the Endocrine SystemMaytansinol
57103-68-1You may like
- Maytansinol 57103-68-1
-
- $110.00 / 20mg
- 2022-02-14
- CAS:57103-68-1
- Min. Order: 20mg
- Purity: ≥98%
- Supply Ability: 1000.00 kgs
- Maytansine
-

- $100.00 / 1KG
- 2021-12-04
- CAS:57103-68-1
- Min. Order: 100g
- Purity: 99.99%
- Supply Ability: 5ton/Month
- MAYTANSINOL
-

- $15.00 / 1KG
- 2021-07-13
- CAS:57103-68-1
- Min. Order: 1KG
- Purity: 99%+ HPLC
- Supply Ability: Monthly supply of 1 ton




