- Maytansinol
-

- $30.00 / 1mg
-
2024-11-19
- CAS:57103-68-1
- Min. Order:
- Purity: 99.71%
- Supply Ability: 10g
- Maytansinol 57103-68-1
-
- $110.00 / 20mg
-
2022-02-14
- CAS:57103-68-1
- Min. Order: 20mg
- Purity: ≥98%
- Supply Ability: 1000.00 kgs
- Maytansine
-

- $100.00 / 1KG
-
2021-12-04
- CAS:57103-68-1
- Min. Order: 100g
- Purity: 99.99%
- Supply Ability: 5ton/Month
Related articles - Preparation of Maytansinol
- Maytansinol is an expensive starting material and its precursor sources are limited to fermentation processes. Maytansinol inh....
- Jan 25,2022
|
| | Maytansinol Basic information |
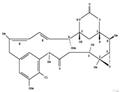
| Product Name: | Maytansinol | | Synonyms: | Maytansine, O3-de2-(acetylmethylamino)-1-oxopropyl-;MAYTANSINOL;3-O-De[2-[methyl(acetyl)amino]-1-oxopropyl]maytansine;Maytansine,3-O-de[2-;3-o-de(2-(acetylMethylaMino)-1-oxopropyl)Maytansine;ansaMitocin p 0;antibiotic c 15003p;Maytansine,3-O-de[2-(acetylMethylaMino)-1-oxopropyl]- | | CAS: | 57103-68-1 | | MF: | C28H37ClN2O8 | | MW: | 565.05 | | EINECS: | | | Product Categories: | Anti-cancerIntermediates & Fine Chemicals;PEG Product | | Mol File: | 57103-68-1.mol |  |
| | Maytansinol Chemical Properties |
| Melting point | 205-207℃ | | Boiling point | 835.8±65.0 °C(Predicted) | | density | 1.34±0.1 g/cm3(Predicted) | | storage temp. | 2-8°C | | solubility | Chloroform (Slightly), Methanol (Slightly) | | form | Solid | | pka | 9.89±0.70(Predicted) | | color | Off-White to Orange |
| | Maytansinol Usage And Synthesis |
| Description | Maytansinol (Ansamitocin P-0) is an inhibitor of microtubule assembly that induces microtubule disassembly in vitro. | | Uses | Maytansinol is used in the preparation of site-specific trastuzumab maytansinoid antibody-drug conjugates with improved therapeutic activity. References Pillow, T.H., et. al.: J. Med. Chem., 57, 7890 (2014) | | Biological Activity | Maytansinol is an ansamacrolide originally isolated from P. verrucose that has antimitotic and anticancer activities. It inhibits polymerization and induces depolymerization of bovine brain tubulin with EC50 values of 12 and 43 μM, respectively. Maytansinol inhibits sea urchin egg mitosis when used at a concentration of 10 μM and decreases proliferation of KB nasopharyngeal cancer cells (EC50 = 0.19 μg/ml). | | Mode of action | Maytansinol (1 b) was first obtained by Kupchan et?al. both by isolation from Putterlickia verrucose and chemical removal of the acyl group from the hydroxy group at the C3 position. It showed weaker inhibitory activity on tubulin polymerization than maytansine, thus implying that the ester moiety at the C3 position of ansamitocins, maytansine, and maytansinoids plays an important role for biological activity and cell permeability. In fact, it has just recently been found that the carbonyl oxygen atom of the ester moiety forms a strong intramolecular interaction with the hydroxy group at position 9, fixing the bioactive conformation. Maytansinol has been regarded as a valuable precursor because acylation allows the preparation of both natural and new semisynthetic maytansinoids, differing in the ester side-chain substituents (Scheme 1). The acylation reaction of maytansinol is a crucial step in the preparation of maytansinoid ADCs or nanoparticles, constituting an uprising class of targeted cancer therapeutics. A few attempts to conjugate maytansinoids to peptides by this reaction have also been made very recently. Furthermore, considering that the maytansine binding site is one of the most recently identified and least explored on tubulin, acylation of maytansinol may serve for the preparation of useful molecular probes to better understand the structure-activity relationships of maytansinoids or to identify new maytansine-site ligands.

Structure of maytansine (1 a), maytansinol (1 b), and the generic acylation reaction of maytansinol. |
| | Maytansinol Preparation Products And Raw materials |
|