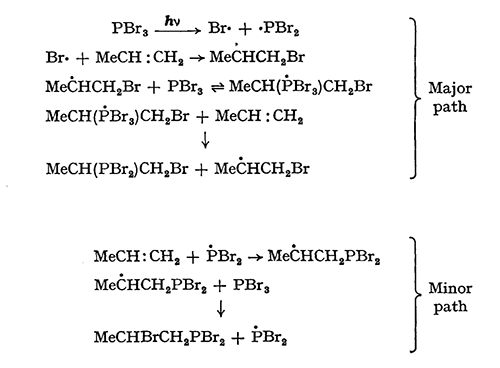Reaction products of polyisobutylene
Jun 8,2022
Background
Polyisobutylene (PIB) is a polymer prepared from isobutylene by positive ion polymerization, and its molecular weight can range from hundreds to millions. It is a typical saturated linear polymer. The main body of the molecular chain does not contain double bonds, and there is no long branched chain. Its structural unit is - (ch2-c (CH3) 2) -, in which there is no asymmetric carbon atom, and the structural unit is connected by a regular sequence from the first to the last[1].

Picture 1 Polyisobutylene liquid
Properties
The most common hydrocarbon tail is polyisobutylene (PIB). Polyisobutylene is used because it has good oil solubility, and it is a good thickener. It decomposes cleanly when heated, has good oxidative stability, and is easily functionalized. Polyisobutylene is made via cationic polymerization [2]. Initiation and propagation steps are shown for an aluminum trichloride catalyzed polymerization of isobutylene. A cation/anion complex is formed when the catalyst interacts with a co-catalyst – water in this case. This initiation complex then reacts with isobutylene to initiate growth of a polymer chain. Chain transfer to monomer followed by a series of rearrangements leads to the product with a variety of end groups .
The cationic polymerization
The cationic polymerization of isobutylene can also be catalyzed by boron trifluoride. Reaction temperatures are often lower with this catalyst system, and terminal rearrangements are much less prominent. The type of polymer produced using this catalyst is referred to as high vinylidene or highly reactive polyisobutylene due to the high level of terminal vinylidene end groups present. The distribution of olefin end groups is very important in determining the reaction conditions used in the next step of dispersant synthesis, which is to react the polymer with a connector, most commonly maleic anhydride. Another very important characteristic of the polyisobutylene product is the molecular weight of the polymer. Commercially available grades of both conventional and high vinylidene
Molecular weights
PIB are typically 900–1,000 Mn (described as low molecular weight) and 2,000–2,300 Mn (described as high molecular weight). Other molecular weights are available, but less commonly used in dispersant synthesis. The choice of molecular weight can be important for the final performance of the molecule as a dispersant. If the tail length is too short, the dispersant will not act properly via the steric mechanism to solubilize contaminant particles and prevent them from agglomerating. If the tail is too long, then the activity of the dispersant is diluted by the long hydrocarbon tail. The most common connector is maleic anhydride.
Reaction products
The reaction product of polyisobutylene with maleic anhydride is often called a polyisobutylene succinic anhydride or PIBSA. The end group distribution of the PIB dictates the reaction conditions used to convert the PIB to PIBSA [3]. For conventional PIB, which is com[1]posed of mainly trisubstituted and tetrasubstituted olefins, chlorine is often used to promote the reaction between the polymer and maleic anhydride [3]. Chlorine adds across the double bond. This addition is followed by the loss of two equivalents of HCl, in successive dehydrohalogenation reactions. Finally, a diene intermediate is formed that reacts with maleic anhydride via a Diels-Alder mechanism to form the final PIBSA product. In this reaction, a new olefin is formed, and the reaction series can repeat itself so that two moles of maleic anhydride can react with one mole of polymer.
Phenol can also serve as a connector between polyisobutylene and amines. Phenol reacts with polyisobutylene in a Friedel-Crafts alkylation [3]. Polyisobutylene with high levels of either the terminal vinylidene olefin or the trisubstituted beta isomer can react with phenol more efficiently than polyisobutylene with high levels of tri- and tetrasubstituted olefins. The tertiary catons formed from the terminal vinylidene and trisubstituted beta isomers are less sterically hindered compared to the cations that would form from tri- and tetrasubstituted polymers.
Reference
1 Kunal K, Paluch M, Roland C M, et al. Polyisobutylene: A most unusual polymer[J]. Journal of Polymer Science Part B: Polymer Physics, 2008, 46(13): 1390-1399.
2 Puskas J E, Chen Y, Dahman Y, et al. Polyisobutylene‐based biomaterials[J]. Journal of Polymer Science Part A: Polymer Chemistry, 2004, 42(13): 3091-3109.
3 Fox Jr T G, Flory P J. Further studies on the melt viscosity of polyisobutylene[J]. The Journal of Physical Chemistry, 1951, 55(2): 221-234.
- Related articles
- Related Qustion
- Polyisobutylene: Industrial Production and Biomedical Application Aug 26, 2024
Polyisobutylene is polymerized with catalysts for specific molecular weights, used in biomedical fields for advanced elastomers, vascular grafts, drug delivery, and enhancing bone cement's properties.
- Polyisobutylene (PIB) Jan 11, 2022
Polyisobutylene (PIB) is a very versatile, non-toxic, water-white viscous liquid and has the ability to increase tackiness, to provide water-repellency, to improve viscosity-index and it provides excellent electrical insulation.
Phosphorus tribromide, also known as phosphorus bromide, is a colorless liquid with a pungent odor, fumes violently in the air, and is poisonous.....
Jun 8,2022APIITP was originally purified and identified based on the stimulatory effect on chloride ion transport measured as changes in short circuit current through the epithelium of the ileum.Its complete primary structure was determined by RT-PCR-ba....
Jun 10,2022Biochemical EngineeringPolyisobutylene
9003-27-4You may like
- Polyisobutylene
-

- $4.00 / 1kg
- 2024-11-16
- CAS:9003-27-4
- Min. Order: 180kg
- Purity: 99%
- Supply Ability: 5000kg/day
- Polyisobutylene
-

- $40.00 / 1kg
- 2024-11-15
- CAS:9003-27-4
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 20ton
- POLYISOBUTYLENE
-

- $6.00 / 1kg
- 2024-11-15
- CAS:9003-27-4
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 2000KG/Month