Synthesis of Ozanimod Hydrochloride
Dec 28,2023
Synthesis of Ozanimod Hydrochloride
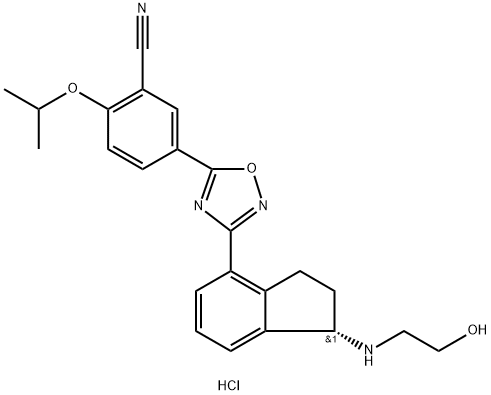
Ozanimod Hydrochloride is prepared from the eastern fragment joined to the western fragment to form a central oxadiazole ring and through a chemical reaction. The specific synthesis steps are as follows:
Step 1: Preparation of Eastern Fragment
Protection of the ketone 184 furnished cyclic ketal 185 in 62% yield. Treatment with hydroxylamine hydrochloride afforded eastern fragment 186 in quantitative yield.
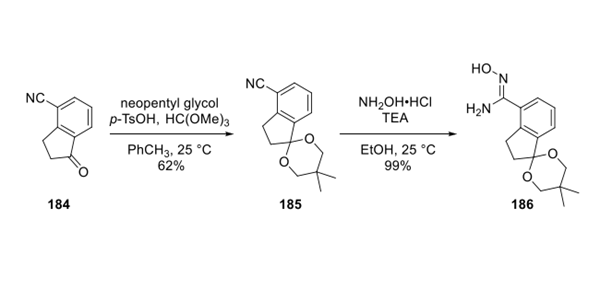
Step 1: Preparation of Ozanimod Hydrochloride
Next, carboxylic acid 187 (western fragment) was activated with CDI and coupled with eastern fragment 186 to form oxadiazole 189. The crude material was used without further purification in the subsequent ketal deprotection, which delivered ketone 190 in 76% yield from 187. Condensation of ketone 190 with 2-aminoethanol under DeanStark conditions afforded imine 191 in 96% yield. Chiral ruthenium complex 192 catalyzes the reduction of imine 190 to ozanimod 193 in high enantiomeric excess. Finally, salt formation with methanolic HCl produces ozanimod hydrochloride in excellent yield.
- Related articles
- Related Qustion
2-Chlorobenzaldehyde, a CS metabolite with moderate lipophilicity, is absorbed through the skin, eliminated via urine, and poses neurotoxic, dermatotoxic, and inhalation risks to humans.....
Dec 28,2023APIRisdiplam was approved by the USFDA for the treatment of spinal muscular atrophy caused by a mutation leading to a deficiency in the survival motor neuron (SMN) protein.....
Dec 28,2023Biochemical EngineeringOzanimod hydrochloride
1618636-37-5You may like
Ozanimod hydrochloride manufacturers
- Ozanimod hydrochloride
-

- $42.00 / 1mg
- 2025-10-23
- CAS:1618636-37-5
- Min. Order:
- Purity: 99.81%
- Supply Ability: 10g