The important applications of 4-chlorophenylacetone
Oct 24,2022
4-Chlorophenylacetone is a chemical raw material and pharmaceutical intermediate with important application value. It has very important applications in modern pharmaceutical, chemical and biological fields [1-3]. 4-Chlorophenylacetone is a common ketone compound. 4-Chlorophenylacetone appears as gray white block crystals. 4-chlorophenylacetone has certain toxicity. When it contacts with skin and mucous membrane, 4-chlorophenylacetone will cause inflammation, so pay attention to safety when using 4-chlorophenylacetone. This chemical is harmful to environment. It should be prevented from contacting groundwater, waterways or sewage systems. Without government permission, 4-chlorophenylacetone should not be discharged into the surrounding environment.;When 4-chlorophenylacetone was used as the substrate, 4-chlorobenzaldehyde was obtained as the major product in 72% GC yield bsp;

Picture 1 4-chlorophenylacetone powders
Research
In recent years, a large number of controlled substance analogs with high structural variety have been widely distributed as noncontrolled alternatives and have been detected in forensic samples. Because of the frequent appearance of new analogs with displacement of a functional group or an alkyl chain, structural differentiation of these new drugs is necessary in forensic laboratories for legal control against drug abuse. 4-Chlorophenylacetone was first reported as a selective serotoninergic depleting agent in rat brains. Chlorinated analogs of amphetamine were subsequently synthesized and examined as appetite depressants. It was eventually concluded that these analogs were not clinically useful and produced less central-nervous system stimulation than amphetamine or methamphetamine. After these reports were published, many researchers studied 4-chlorophenylacetone as factors of selective serotonin (5-hydroxytriptamine, 5-HT) diminution and as monoamine oxidase (MAO) inhibitors in brain nerve terminals. It was also reported that 4-chlorophenylacetone cause long-lasting depletion in the level of brain 5-HT and 5-hydroxyindole-3-acetic acid without depleting brain noradrenaline.
Preparation of 4-chlorophenylacetone
At present, there are three main synthetic routes for 4-chlorophenylacetone. The first is to synthesize 4-chlorophenylacetone with sodium p-chlorobenzenesulfonate and acetone as raw materials, with a yield of about 54%. The second synthesis route is to synthesize 4-chlorophenylacetone with p-chlorobenzenesulfonyl chloride and chloroacetone as raw materials. The third synthesis route is to synthesize 4-chlorophenylacetone from chloroacetone.
Application of 4-chlorophenylacetone
With the continuous expansion of research and development of major chemical plants and pharmaceutical enterprises at home and abroad on structural transformation of new, efficient and low toxic agricultural and pharmaceutical products, the demand for types of pharmaceutical raw materials is also increasing. It is very important to find new compounds as starting materials. In order to further expand the research scope of new products such as medicine and chemical industry with 4-chlorophenylacetone as the starting material, we have synthesized a new compound, 2-methyl-4-chlorophenylacetone, as the starting material of drug structure transformation. The synthetic route using N-m-tolyl acetamide as raw material was determined to be an economical and industrial process[1].
The effective component of dapoxetine hydrochloride tablets is dapoxetine, which is a selective serotonin reuptake inhibitor. Such drugs as dapoxetine are widely used in the treatment of adult male premature ejaculation (PE). There are chemicals such as serotonin and dopamine in the sexual desire center of the brain, which can transmit strong ejaculation impulse, while dapoxetine can interfere with and inhibit the above two chemicals, so as to delay the ejaculation time. 4-chlorophenylacetone is an important chemical material, which can be used as raw material of many chemicals. Dapoxetine hydrochloride is prepared by adding 4-chlorophenylacetone and methanol to 250 ml-three-necked flask, adding potassium borohydride, controlling the temperature of the reaction solution to 0-5 degrees C, reacting at 5-15 degrees C for 2 hours, adding methanol, distilling under reduced pressure, adding water, extracting with ethyl acetate three times, combining organic layers, drying, and distilling to remove ethyl acetate under reduced pressure at 50 degrees C[2].
Reference
1 Yu, C.J.,Kayyem, Jon Faiz,Gozin, Michael, et al.Soluble ferrocene conjugates for incorporation into serf-assembled monolayers[J].The Journal of Organic Chemistry.1999,64(6).2070-2079.
2 HU H; LI J; LU H; TANG Z. Preparation of dapoxetine hydrochloride involves adding crude dapoxetine hydrochloride and isopropanol to three-necked flask, heating to reflux, cooling, filtering, washing resultant filter cake with ethyl acetate, and drying. CN106748817(A).
3 Wu Yin Synthesis of a new cannabinoid receptor antagonist rimonabant [D]. Hebei Medical University, 2007
- Related articles
- Related Qustion
NADPH mainly acts as the coenzyme of dehydrogenase and acts as a hydrogen transmitter in the enzymatic reaction.....
Oct 24,2022API7, 8-Dihydroxyflavone is a compound with biological effects. 7,8-DHF has a strong therapeutic effect on Alzheimer's disease and can inhibit obesity by activating muscle TrkB.....
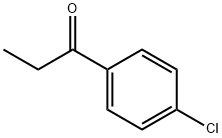
Oct 24,2022Inhibitors4'-Chloropropiophenone
6285-05-8You may like
4'-Chloropropiophenone manufacturers
- 4'-Chloropropiophenone
-

- $10.00 / 1ASSAYS
- 2025-11-17
- CAS:6285-05-8
- Min. Order: 1ASSAYS
- Purity: 99%
- Supply Ability: 10 ton
- 4'-Chloropropiophenone
-

- $0.00 / 1kg
- 2025-11-17
- CAS:6285-05-8
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 1000
- 4'-Chloropropiophenone
-

- $0.00 / 1kg
- 2025-11-17
- CAS:6285-05-8
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 1000