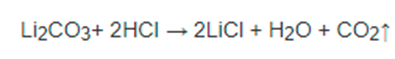The toxicity of 2-bromoethanol
Apr 11,2022
Introduction
2-Bromoethanol is a colorless or light yellow hygroscopic liquid with relatively stable and toxic chemical properties[1]. It plays a role in industrial and chemical experiments. It shall be stored in a ventilated, low-temperature and dry warehouse and separated from oxidants, acids and food additives. It is miscible with water and forms an azeotrope with water. The boiling point is 99.1 ℃ (101.35kpa). The aqueous solution has a sweet burning taste. It can be miscible with most organic solvents such as ethanol and ether, but insoluble in petroleum ether. The hydrolysis of aqueous solution can be accelerated when it meets acid, alkali and heating.

Picture 1 2-Bromoethanol liquid
Toxicity of 2-bromoethanol
2-Bromoethanol can damage human body[1]. The invasive ways of 2-bromoethanol include inhalation, ingestion and percutaneous absorption. 2-Bromoethanol has strong irritation to mucosa, upper respiratory tract, eyes and skin. Inhalation of 2-bromoethanol can cause death due to spasm, inflammation and edema of larynx and bronchus, chemical pneumonia and pulmonary edema. The manifestations of 2-bromoethanol poisoning include burning sensation, cough, wheezing, laryngitis, shortness of breath, headache, nausea and vomiting.
Environmental behavior of 2-bromoethanol
Mouse oral TD50: 43mg / kg / 80w-c, tumor ambiguous tumor, RTECS standard gastrointestinal tumor; TD50 of mice via abdominal cavity: 150 mg / kg / 8w-i, RTECS standard of tumor tumor, lung, chest or respiratory tumor. Microbial organism test system mutation: bacteria Salmonella typhimurium: 1 mg / plate; DNA repairtest system: bacteria - Escherichia coli: 10 μ mol/plate; Mutant microorganismtest system: bacteria Klebsiella pneumoniae: 15 mmol / L. The minimum lethal concentration of 2-bromoethanol injected intraperitoneally into mice was 80mg / kg. 2-bromoethanol is flammable in case of open fire. It burns and decomposes under high heat to produce toxic gas. Combustion (decomposition) products: carbon monoxide, carbon dioxide, hydrogen bromide. Gas chromatography is generally used to monitor 2-bromoethanol in the laboratory.
Emergency treatment of 2-bromoethanol leakage
Quickly evacuate the personnel in the leakage contaminated area to the safe area, isolate them and strictly restrict their access[2]. Cut off the fire source. It is recommended that emergency treatment personnel wear self-contained positive pressure respirator and anti-virus clothing. Do not directly cut off the leakage source as much as possible to prevent entering the restricted space such as sewer and flood drainage ditch. In case of small leakage of 2-bromoethanol, flush the leakage area with a large amount of water, dilute the washing water and put it into the wastewater system. When a large amount of leakage occurs, build a dike or dig a pit for reception. And cover with foam to reduce steam disaster. Transfer to tank car or special collector by pump, recycle or transport to waste treatment site for disposal.
First aid measures for 2-bromoethanol
In case of skin contact with 2-bromoethanol, immediately remove the contaminated clothes and rinse with a large amount of flowing water for at least 15 minutes. See a doctor. In case of eye contact, immediately lift the eyelids and rinse with a large amount of flowing water or normal saline for at least 15 minutes. See a doctor. When inhaling 2-bromoethanol, leave the site quickly to a place with fresh air. Keep the respiratory tract unobstructed. If breathing is difficult, give oxygen. If breathing stops, carry out artificial respiration immediately and seek medical attention. Those who take 2-bromoethanol by mistake gargle with water and drink milk or egg white. See a doctor. The fire extinguishing method of 2-bromoethanol is to move the container from the fire site to an open place as far as possible. Keep the containers in the fire site cool with water until the end of fire fighting. Fire extinguishing agents generally use mist water, anti foaming foam, dry powder and carbon dioxide.
Reference
1 Kaphalia B S, Ansari G A S. Hepatic fatty acid conjugation of 2‐chloroethanol and 2‐bromoethanol in rats[J]. Journal of biochemical toxicology, 1989, 4(3): 183-188.
2 Hintsa E J, Zhao X, Lee Y T. Photodissociation of 2‐Bromoethanol and 2‐Chloroethanol at 193 nm[J]. The Journal of chemical physics, 1990, 92(4): 2280-2286.
- Related articles
- Related Qustion
Acetamide is an organic compound with molecular formula C2H5NO[1], molecular weight 59.07, density 1.159.....
Apr 11,2022APILithium chloride appears as colorless crystals or powder. Low toxicity. Lithium chloride is a metal chloride salt with a Li(+) counterion. It has a role as an antimanic drug and a geroprotector.....
Apr 11,2022Drugs2-Bromoethanol
540-51-2You may like
- 2-Bromoethanol
-

- $0.00 / 1kg
- 2024-10-25
- CAS:540-51-2
- Min. Order: 1kg
- Purity: 0.99
- Supply Ability: 20 tons
- 2-Bromoethanol
-

- $1.50 / 1g
- 2023-07-27
- CAS:540-51-2
- Min. Order: 1g
- Purity: 99.0% Min
- Supply Ability: 100 Tons
- 2-Bromoethanol
-

- $0.00 / 25KG
- 2023-07-22
- CAS:540-51-2
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 50000KG/month