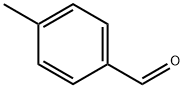
p-Tolualdehyde: Applications in Analytical Chemistry and its Production Method
Jul 16,2024
General Description
p-Tolualdehyde is a crucial compound in analytical chemistry, aiding in the quantification of hydrazine and acetylhydrazine in biological samples through derivatization for improved detection using HPLC-MS/MS. Its use enhances separation and detection on a C18 column. In clinical settings, p-Tolualdehyde plays a vital role in monitoring patients treated with isoniazid for tuberculosis, contributing to understanding pharmacokinetics and ensuring patient safety. The innovative production of p-Tolualdehyde from p-xylene using a photoinduced electron transfer method exhibits high selectivity, avoiding byproducts. Additionally, a related method generates p-Tolualdehyde and hydrogen peroxide simultaneously, showcasing dual production benefits for economic value and practicality.

Figure 1. p-Tolualdehyde
Applications in Analytical Chemistry
Introduction to p-Tolualdehyde in Derivatization
p-Tolualdehyde plays a pivotal role in the field of analytical chemistry, particularly in the enhancement of detection methods for specific compounds within biological samples. A notable application of p-Tolualdehyde is its use in the derivatization process for the quantification of hydrazine and acetylhydrazine in human plasma. This process significantly improves the detectability and measurement accuracy of these compounds by high-performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS). Through the reaction facilitated by p-Tolualdehyde, the compounds hydrazine and acetylhydrazine can be converted into more detectable forms, thereby enabling their precise quantitation in medical and research settings.
Methodology and Optimization of p-Tolualdehyde Derivatization
The methodology utilizing p-Tolualdehyde involves a straightforward derivatization reaction facilitated by ultrasonic manipulation for 40 minutes. This approach leverages the chemical properties of p-Tolualdehyde to bind with hydrazine derivatives, forming products that are more amenable to separation and detection using a C18 column and gradient elution. The optimization of this process has been focused on achieving excellent separation and quantifiable detection limits, with p-Tolualdehyde enhancing the mass spectrometry signals of the derivatives. Specifically, the mass transition ion-pairs were carefully selected to monitor the derivatized forms of hydrazine and acetylhydrazine, demonstrating the versatility and effectiveness of p-Tolualdehyde in improving analytical outcomes.
Clinical Application and Impact of p-Tolualdehyde
The practical application of the p-Tolualdehyde-based method has proven to be profoundly beneficial in clinical settings, particularly for monitoring patients undergoing treatment with isoniazid for pulmonary tuberculosis. The ability of p-Tolualdehyde to facilitate the detection of low concentrations of hydrazine and acetylhydrazine has enabled clinicians to better understand the pharmacokinetics and potential toxicology of these compounds in patients. This has significant implications for patient safety and the optimization of therapeutic strategies. The developed HPLC-MS/MS method, enhanced by p-Tolualdehyde, not only offers a high degree of sensitivity and specificity but also demonstrates the potential of chemical derivatization in expanding the capabilities of modern medical diagnostics. 1
Production Method
Photoinduced Electron Transfer Method
The production of p-Tolualdehyde via the selective oxygenation of p-xylene showcases an innovative approach using photoinduced electron transfer. This method involves the irradiation of p-xylene with visible light, which facilitates the transfer of electrons from p-xylene to the singlet excited state of the 10-methyl-9-phenylacridinium ion. The high selectivity of this photocatalytic process results in p-Tolualdehyde being the sole product. This selective process demonstrates the efficiency of utilizing specific catalytic conditions and light to drive chemical transformations, thereby producing p-Tolualdehyde in a highly controlled and efficient manner. 2
Mechanism of High Selectivity in p-Tolualdehyde Production
The remarkable selectivity in the production of p-Tolualdehyde from p-xylene is primarily attributed to the specific mechanism of photoinduced electron transfer. This method ensures that p-xylene is transformed into p-Tolualdehyde without the byproducts typically associated with other oxidation methods. The process hinges on the precise conditions under which the electron transfer occurs, emphasizing the role of the 10-methyl-9-phenylacridinium ion in mediating this transformation. The discussion around this method centers on understanding how the electron transfer contributes to the exclusive formation of p-Tolualdehyde, thereby optimizing the yield and purity of the final product.
Concurrent Production and Hydrogen Peroxide
In a related photocatalytic method, p-Tolualdehyde is produced simultaneously with hydrogen peroxide, showcasing a dual production strategy that enhances the utility of the process. This method utilizes the 9-mesityl-2,7,10-trimethylacridinium ion (Me(2)Acr(+)-Mes) under photoirradiation to initiate the photoinduced electron transfer, leading to the oxidation of p-xylene and reduction of oxygen. The efficient conversion of p-xylene into p-Tolualdehyde, along with the formation of hydrogen peroxide, highlights the potential for integrated production processes in chemical manufacturing. This approach not only yields p-Tolualdehyde but also produces valuable by-products, enhancing the overall economic and practical value of the photocatalytic process. 3
Reference
1. Song L, Gao D, Li S, Wang Y, Liu H, Jiang Y. Simultaneous quantitation of hydrazine and acetylhydrazine in human plasma by high performance liquid chromatography-tandem mass spectrometry after derivatization with p-tolualdehyde. J Chromatogr B Analyt Technol Biomed Life Sci. 2017; 1063: 189-195.
2. Ohkubo K, Fukuzumi S. 100 selective oxygenation of p-xylene to p-tolualdehyde via photoinduced electron transfer. Org Lett. 2000; 2(23): 3647-3650.
3. Ohkubo K, Mizushima K, Iwata R, Souma K, Suzuki N, Fukuzumi S. Simultaneous production of p-tolualdehyde and hydrogen peroxide in photocatalytic oxygenation of p-xylene and reduction of oxygen with 9-mesityl-10-methylacridinium ion derivatives. Chem Commun (Camb). 2010; 46(4): 601-603.
- Related articles
- Related Qustion
D-Tartaric acid boosts biosensor sensitivity for Vibrio parahaemolyticus, vital for food safety. Its eco-friendly biological synthesis promises sustainable production with confirmed purity.....
Jul 16,2024API4-Bromofluorobenzene is pivotal in organic electronics, synthesis, and analytical chemistry, yet requires strict safety protocols due to toxicological risks in handling.....
Jul 16,2024APIp-Tolualdehyde
104-87-0You may like
- Sorbic acid: Benefits and Side effects
Jul 19, 2024
- Uses of Sulfanilic acid
Jul 19, 2024
- Sodium Lauryl Ether Sulfate: Applic....
Jul 19, 2024
- p-Tolualdehyde
-

- $0.00 / 1kg
- 2024-05-11
- CAS:104-87-0
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 500000kg
- p-Tolualdehyde
-
- $5.00 / 1KG
- 2024-04-05
- CAS:104-87-0
- Min. Order: 1KG
- Purity: 98%
- Supply Ability: g-kg-tons, free sample is available
- p-Tolualdehyde
-

- $9.10 / 1KG
- 2023-03-06
- CAS:104-87-0
- Min. Order: 1KG
- Purity: 99.%
- Supply Ability: 10 ton