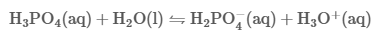Inorganic chemicals is the shortened form of inorganic chemical industry and is an important branch of the chemical industry with natural resources and industrial by-products as raw materials for the production of sulfuric acid, nitric acid, hydrochloric acid, phosphoric acid, soda ash, caustic soda, synthetic ammonia, fertilizer and inorganic salts, etc. This includes sulfuric acid industry, soda industry, the chloro-alkali industry, synthetic ammonia industry, fertilizer industry and mineral industry. Its broad definition also includes the production of inorganic non-metallic materials and fine inorganic product such as ceramics and inorganic pigment. The main raw material of inorganic chemical products are mineral product including sulfur, sodium, phosphorus, potassium and calcium and coal, oil, gas, and air, water and so on. Inorganic chemicals can be traced back to the ancient process of ceramics, alchemy, brewing, dyeing at thousands of years ago. Although with small scale, backward technology and pure manual manipulation, but it is the prototype of inorganic chemicals. For thousands of years, due to the low productivity, it gets slow development. Until the 18th century, it had developed rapidly. In the middle of 18th century, Britain had first applied lead chamber method using saltpeter and sulfur as raw materials to produce sulfuric acid. In 1783, Lu Bulan (France) proposed the soda method using sodium chloride, sulfuric acid, coal as raw materials. In the latter half of the 18th century, the modern chemical industry taking inorganic chemical industry as the main content had began to emerge. In 1841, people began the production of phosphate fertilizer; In 1965 Belgian Solvay realized the industrialization of ammonia soda for production of soda; with the rise of preparing potassium industry in 1870; In 1890, people began to use electrolytic approach for making Cl2 and caustic soda; In 1913, people had achieved the catalytic synthesis
Q:Is it safe to drink baking soda water?
A:It’s usually safe to drink. Some people drink baking soda for indigestion and other purposes, but drinking baking soda can be dangerous and is not suitable for long-term use, use during pregnancy, or
Mar 19,2024 Inorganic chemistryIs AlCl3 ionic or covalent?
AlCl3 is covalent. Aluminum chloride (AlCl3) is a compound that exhibits characteristics of both ionic and covalent bonding, making it somewhat ambiguous in terms of its classification.
Mar 19,2024 Inorganic chemistryWhat is graphite metal?
Graphite is a is a form of carbon and a non-metal but the only non-metal can conduct electricity. It is an excellent conductor of heat and electricity and has the highest natural strength and stiffne
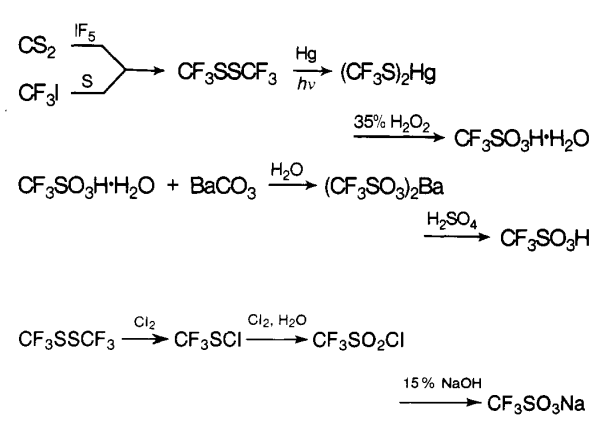
Mar 18,2024 Inorganic chemistryHow to synthesize Trifluoromethanesulfonic acid?
The first reported syntheses of trifluoromethanesulfonic acid appeared in 1954.
Mar 18,2024 Inorganic chemistryUses and Hazards of Bitumen
Bitumen is a primary engineering material, functioning as a binder in road construction and as a weatherproofing membrane in roofing and structural applications.
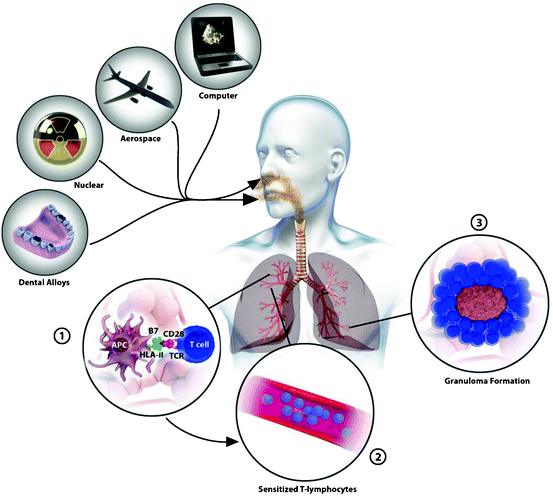
Mar 16,2024 Inorganic chemistryThe safety of Beryllium
Beryllium can elicit a strong cellular immune response in vivo. Chronic beryllium disease is an allergic reaction of the delayed type, in which beryllium appears to act as a hapten that binds to pepti
Mar 16,2024 Inorganic chemistryUses and Health hazard of Asbestos
Asbestos is the common name of several naturally occurring, hydrated silicate minerals that possess a crystalline structure and low thermal conductivity.
Mar 16,2024 Inorganic chemistryBeryllium: Discovery, Physical Properties, and Chemical property
Beryllium, Be, is the first element in the second main group of the periodic table. It is a light metal with a hexagonal-closest-packed (hcp) structure.
Mar 16,2024 Inorganic chemistryUses and safety of high-purity arsenic
The demand for metallic arsenic is limited. It is used in nonferrous alloys, and high-purity arsenic is used in electronic and semiconductor devices.
Mar 16,2024 Inorganic chemistryWhat is the pH value of phosphoric acid?
Phosphoric acid, also known as orthophosphoric acid or phosphoric(V) acid, is a mineral (inorganic) acid having the chemical formula H3PO4. The pH of a 0.15 M solution of phosphoric acid (H3PO4) is m
Mar 15,2024 Inorganic chemistry