A reducing agent (also called a reductant or reducer) is an element (such as calcium) or compound that loses (or "donates") an electron to another chemical species in a redox chemical reaction. Since the reducing agent is losing electrons, it is said to have been oxidized. If any chemical is an electron donor (reducing agent), another must be an electron recipient (oxidizing agent). A reducing agent is oxidized because it loses electrons in the redox reaction. Thus, reducing agents "reduce" (or, seen another way, are "oxidized" by) oxidizing agents, and oxidizers "oxidize" (that is, are "reduced" by) reducers. In their pre-reaction states, reducers have more electrons (that is, they are by themselves reduced) and oxidizers have fewer electrons (that is, they are by themselves oxidized). A reducing agent typically is in one of its lower possible oxidation states and is known as the electron donor. Examples of reducing agents include the earth metals, formic acid, oxalic acid, and sulfite compounds.
Sodium thioglycolate: Synthesis, Application and Toxicity Studies
Sodium thioglycolate is one of the salts of thioglycolic acid commonly used in consumer products to wave, straightenor remove hair.
Mar 19,2025 Reducing agentHow does ammonium thioglycolate affect hair?
Ammonium thioglycollate is a key ingredient in many hair care products, such as hair relaxers, perms, and straightening creams.
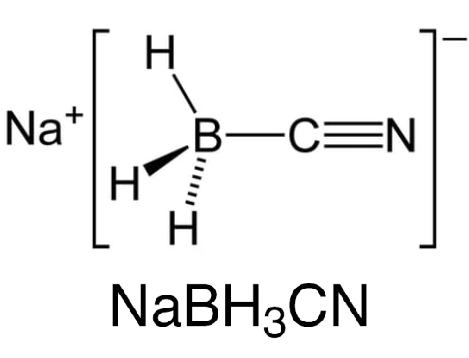
Nov 6,2024 Reducing agentOne of the reductants for reductive amination: sodium cyanoborohydride
Sodium cyanoborohydride is a selective reducing agent used for various chemical reductions, including aldehydes, ketones, oximes, enamines, reductive aminations of aldehydes and ketones, and reductive
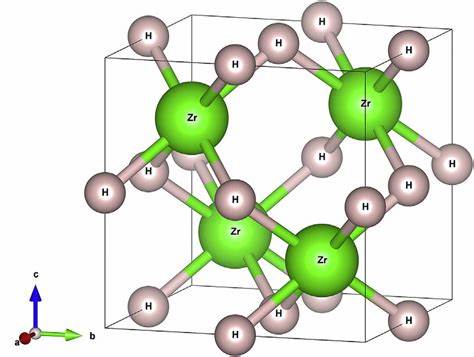
May 8,2024 Reducing agentZirconium hydride:Structure, Production and Uses
Zirconium hydride is used in the nuclear industry as a moderator for thermal neutrons, especially in light water reactors and fast breeder reactors.
Oct 21,2023 Reducing agentLithium Tetrahydridoaluminate:A powerful nucleophilic reducing agent
Lithium aluminum hydride is a powerful nucleophilic reducing agent. It reduces almost all functional groups, although isolated double and triple bonds in alkenes and alkynes are generally not attacked
Oct 21,2023 Reducing agentApplication of Poly(methylhydrosiloxane)
Poly(methylhydrosiloxane) is a reducing agent. For example, it can be used for the reduction of esters to alcohols as well as the reduction of aldehydes and ketone. It can also be used for the reducti
Aug 14,2019 Reducing agent