| Identification | More | [Name]
1-Propanesulfonyl chloride | [CAS]
10147-36-1 | [Synonyms]
1-PROPANESULFONYL CHLORIDE
PROPANE-1-SULFONYL CHLORIDE
PROPANE SULFONYL CHLORIDE
1-Propanesulfonyl Chloride, Pract.
propanesulphonyl chloride
Propane-1-sulfonic acid chloride | [EINECS(EC#)]
233-414-7 | [Molecular Formula]
C3H7ClO2S | [MDL Number]
MFCD00007462 | [Molecular Weight]
142.6 | [MOL File]
10147-36-1.mol |
| Safety Data | Back Directory | [Hazard Codes ]
C | [Risk Statements ]
R14:Reacts violently with water.
R34:Causes burns.
R36:Irritating to the eyes. | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection .
S45:In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) . | [RIDADR ]
UN 3265 8/PG 2
| [WGK Germany ]
3
| [F ]
10-19-21 | [TSCA ]
Yes | [HazardClass ]
8 | [PackingGroup ]
III | [HS Code ]
29049090 |
| Hazard Information | Back Directory | [Chemical Properties]
Light yellow liquid | [Uses]
1-Propanesulfonyl chloride was used in synthesis of 1-alkyl-3-methylimidazolium alkanesulfonate ionic liquids. | [Synthesis]
2,4,6-Trichloro-[1,3,5]-triazine (TCT) was added at room temperature to a solution of propane-1-sulfonic acid in acetone dry, followed by NEt3 dropwise. The resulting mixture was irradiated to 80 °C (50 W of MW power) for 20 min in a sealed tube (10 mL pressure-rated reaction vial) in a self-tuning single-mode irradiating synthesizer. The mixture was cooled rapidly to room temperature by passing compressed air through the microwave cavity for 1 min. After cooling to room temperature, the precipitate was filtered off on Celite to afford 1-Propanesulfonyl chloride[1].
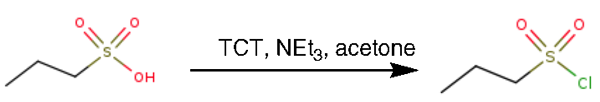
| [References]
[1] Lidia De Luca, Giampaolo Giacomelli. “An Easy Microwave-Assisted Synthesis of Sulfonamides Directly from Sulfonic Acids.” The Journal of Organic Chemistry 73 10 (2008): 3967–3969. |
|
|