| Identification | More | [Name]
Benzonatate | [CAS]
104-31-4 | [Synonyms]
2-[2-[2-[2-[2-[2-[2-[2-(2-methoxyethoxy)ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethyl-4-butylaminobenzoate
2,5,8,11,14,17,20,23,26-NONAOXAOCTACOSAN-28-YL P-(BUTYLAMINO)-BENZOATE
BENZOIC ACID,4-(BUTYLAMINO)-,2,5,8,11,14,17,20,23,26-NONAOXAOCTACOS-28-YL ESTER
BENZONATATE
4-(Butylamino)benzoicacid3,6,9,12,15,18,21,24,27-nonaoxao-ctacos-1-ylester
benzoicacid,4-(butylamino)-,3,6,9,12,15,18,21,24,27-nonaoxaoctacos-1-yles
benzononantin
benzononatine
exangit
km65
p-(butylamino)-benzoicaci2-(2-(2-(2-(2-(2-(2-(2-(2-methoxyethoxy)ethoxy)e
p-(n)-butylaminobenzoesaeure-(nonaaethylenglykol-monomethylaether)-ester
polyethyleneglycol-p-n-butylaminobenzoatemethylester
tesalon
tessalin
tessalon
tessalon-ciba
thoxy)ethoxy)ethoxy)ethoxy)ethoxy)ethoxy)ethylester
ventussin
ventussin-loz | [EINECS(EC#)]
203-194-7 | [Molecular Formula]
C30H53NO11 | [MDL Number]
MFCD00072060 | [Molecular Weight]
603.74 | [MOL File]
104-31-4.mol |
| Chemical Properties | Back Directory | [Boiling point ]
649.0±55.0 °C(Predicted) | [density ]
1.096±0.06 g/cm3(Predicted) | [storage temp. ]
Refrigerator, under inert atmosphere | [solubility ]
Chloroform (Slightly), Methanol (Slightly) | [form ]
Oil | [pka]
2.20±0.39(Predicted) | [color ]
Light Yellow to Light Brown | [Usage]
Antitussive | [BCS Class]
3? | [CAS DataBase Reference]
104-31-4(CAS DataBase Reference) |
| Hazard Information | Back Directory | [Description]
Benzonatate is a reversible voltage-gated sodium channel blocker.1 It blocks Nav1.7 currents in a concentration- and voltage-dependent manner (IC50s = 5.9 and 1.4 μM at holding potentials of -100 and -70 mV, respectively) and inhibits action potential firing in catecholamine A differentiated (CAD) cells. Benzonatate also blocks 80% of Nav1.3 currents in N1E-115 cells when used at a concentration of 100 μM. In vivo, benzonatate (0.85 mg/min) reduces the frequency, but has no effect on the amplitude, of the cough reflex in anesthetized dogs.2 Formulations containing benzonatate have been used as antitussive agents for the treatment of coughs. | [Originator]
Tessalon,Endo (Du Pont),US,1958 | [Uses]
Antitussive | [Definition]
ChEBI: The ester obtained by formal condensation of 4-butylaminobenzoic acid with nonaethylene glycol monomethyl ether. Structurally related to procaine and benzocaine, it has an anaesthetic effect on the stretch sensors in the lungs, and is used as a non-narcoti
cough suppressant. | [Manufacturing Process]
4.42 parts of para-butylamino-benzoic acid ethyl ester are put with 16.0 parts
of a mixture of polyethylene glycol monomethyl ethers, boiling at 180°-220°C
at a pressure of 0.01 mm of mercury, in a closed reaction vessel which is
fitted with an adjustable inlet tube for solvents and a connection for distilling
off in vacuo. In order to dry the mixture completely, it is heated for an hour at
100°-105°C and absolute xylene is introduced under the surface of the
mixture in vacuo at a pressure of 12 mm of mercury. There is thus a constant
stream of xylene steam passing through the whole apparatus, which removes
the last traces of moisture and any other volatile impurities. The xylene is
condensed in a cooler. The whole is cooled to 20°-30°C and 0.06 part of
sodium methylate dissolved in 0.6 part of methanol is added.
Thereupon xylene is introduced again in vacuo at a temperature of 100°-
105°C whereby all the methanol and the ethanol formed during re�esterification evaporates. The re-esterification is continued under these
conditions until a specimen of the reaction mass is clearly soluble in cold
water, which occurs after about 2-3 hours. There is now obtained in almost
quantitative yield the ester of the formula wherein n stands for approximately
7 to 9, which still contains an excess of polyethylene glycol monomethyl ether.
The ester is purified by dissolving in benzene and being washed several times
with a sodium carbonate solution of 5% strength. It is advantageous to
agitate all the washing solutions with fresh benzene. In this distribution
between benzene and sodium carbonate solution the new ester remains in the
benzene, the excess polyethylene glycol monomethyl ether and a small
amount of brown impurities are taken up by the dilute soda solution. By
evaporating the dried and filtered benzene solution there is obtained the new
ester in the form of a colorless to very faintly yellow oil which is easily soluble
in most organic solvents with the exception of aliphatic hydrocarbons. The
new ester is precipitated from aqueous solutions when heated to about 42°C.
but it dissolves again readily on cooling. | [Brand name]
Tessalon (Forest). | [Therapeutic Function]
Antitussive | [Biological Functions]
Benzonatate (TessaIon) is related to the local anesthetic
tetracaine. It anesthetizes the stretch receptors in
the lungs, thereby reducing coughing.Adverse reactions
include hypersensitivity, sedation, dizziness, and nausea. | [Mechanism of action]
It is believed that it acts by two mechanisms: selective anesthesia of irritated receptors in
the lungs and simultaneous suppression of the cough center. | [Synthesis]
Benzonatate, p-butylaminobenzoate 2,5,8,11,14,17,20,23,26-nonaoctacozan-
28-ol (23.2.2), is synthesized by reesterifying the ethyl ester of 4-butylaminobenzoic
acid with the monomethyl ether nonaethylenglycol. It is a structural analog of the
local anesthetic tetracaine.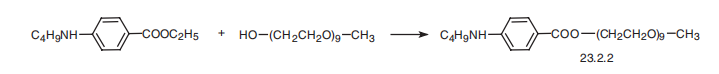
| [storage]
Store at -20°C |
|
|