| Identification | More | [Name]
Glutarimide | [CAS]
1121-89-7 | [Synonyms]
2,6-DIKETOPIPERIDINE
2,6-PIPERIDINEDIONE
GLUTARIMIDE
PIPERIDINE-2,6-DIONE
Glutarimide98%
2,6-Dioxopiperidine | [EINECS(EC#)]
214-340-4 | [Molecular Formula]
C5H7NO2 | [MDL Number]
MFCD00006670 | [Molecular Weight]
113.11 | [MOL File]
1121-89-7.mol |
| Chemical Properties | Back Directory | [Appearance]
white crystalline powder | [Melting point ]
155-157 °C (lit.) | [Boiling point ]
211.82°C (rough estimate) | [density ]
1.2416 (rough estimate) | [refractive index ]
1.4200 (estimate) | [storage temp. ]
Inert atmosphere,Room Temperature | [solubility ]
soluble in Chloroform, DCM | [form ]
Crystals or Crystalline Flakes | [pka]
pKa 11.4 (Uncertain) | [color ]
White | [Water Solubility ]
Soluble in water, hot ethanol and boiling benzene. Insoluble in ether. | [BRN ]
110052 | [InChI]
InChI=1S/C5H7NO2/c7-4-2-1-3-5(8)6-4/h1-3H2,(H,6,7,8) | [InChIKey]
KNCYXPMJDCCGSJ-UHFFFAOYSA-N | [SMILES]
N1C(=O)CCCC1=O | [CAS DataBase Reference]
1121-89-7(CAS DataBase Reference) | [EPA Substance Registry System]
1121-89-7(EPA Substance) |
| Safety Data | Back Directory | [Hazard Codes ]
Xi | [Risk Statements ]
R36:Irritating to the eyes. | [Safety Statements ]
S37/39:Wear suitable gloves and eye/face protection .
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice . | [WGK Germany ]
3
| [RTECS ]
MA4000000
| [HS Code ]
29251995 |
| Hazard Information | Back Directory | [Description]
Glutarimide, also known as Piperidine-2,6-dione or 2,6-Piperidinedione, is a versatile and commonly used organic reagent, classified in pharmacology as a protein synthesis inhibitor compound. It is also an important component of the anti-cancer drug thalidomide. The study reported the synthesis of triazoloquinoxaline derivative (Compound 15), a new potent anticancer drug containing a glutarimide molecule, based on the CRBN binding property of the glutarimide molecule in thalidomide molecule and the cytotoxic activity of triazole-quinoxaline. The IC50s were 9.81 ± 0.7, 15.49 ± 1.2 and 10.09 ± 0.9 μM for hepatocellular carcinoma (HepG2), prostate cancer (PC3) and breast cancer (MCF-7), respectively. It was superior to thalidomide in decreasing NF-κB P65 levels in HepG-2 cells[1]. | [Chemical Properties]
white crystalline powder | [Uses]
Glutarimide acts as an inhibitor of protein synthesis. Further, it is used as a reactant for thionations and biocatalytic asymmetric synthesis of sitagliptin production. It is also employed in the generation of beta-adrenoceptor ligands, enantioselective synthesis of securinega alkaloids and alfa-fluoro-alfa amino amides. In addition to this, it is used in intramolecular amidocyclopropanation reactions. | [Definition]
ChEBI: A dicarboximide that is piperidine which is substituted by oxo groups at positions 2 and 6. | [Preparation]
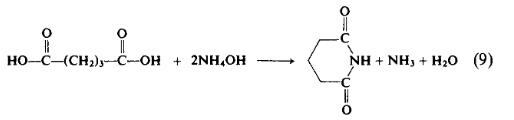
To a flask containing 70 gm (0.53 mole) of glutaric acid is added 150 ml (2.2 mole) of 28% aqueous ammonia. The mixture is set for distillation and heated for 7 hr as the temperature of the mixture rises from 90° to 180°C. The temperature is held at 170-180°C for \\ hr or until the evolution of ammonia ceases. The reaction mixture solidifies on cooling and the product is recrystallized from acetone to afford 37.4 gm (63%), m.p. 145-146°C.
t may be advantageous to preform the ammonium salt of dicarboxylic acids prior to the application of enough heat to form the imide. The preparation of succinimide is a case in point.
| [General Description]
A glutarimide antibiotic, 9-methylstreptimidone, shows antiviral, antitumor and antifungal activities. | [Purification Methods]
Purify it by dissolving 75g in 200mL of H2O, boil for 30minutes with 2g of charcoal, filter, evaporate to dryness and recystallise the residue from 125mL of 95% EtOH to give 70g of white crystals, m 152-154o. It also crystallises from Me2CO (m 163-165o) or EtOH (m 153-154o). The N-bromo derivative (a brominating agent) crystallises from H2O with m 180-185o. [Paris et al. Org Synth Coll Vol IV 496 1963, Beilstein 21 H 382, 21 I 331, 21 II 307, 21 III/IV 4582.] | [References]
[1] MAGED MOHAMMED SALEH AL WARD . Design, synthesis and biological evaluation of newly triazolo-quinoxaline based potential immunomodulatory anticancer molecules[J]. Journal of Molecular Structure, 2023. DOI:10.1016/j.molstruc.2023.137041. |
|
|