| Identification | More | [Name]
DIBENZANTHRONE | [CAS]
116-71-2 | [Synonyms]
DIBENZANTHRONE
VAT BLUE 20
VIOLANTHRONE
5,10-Violanthrenedione
Ahcovat Dark Blue BO
ahcovatdarkbluebo
Amanthrene Dark Blue BO
Amanthrene Supra Dark Blue BO
amanthrenedarkbluebo
amanthrenesupradarkbluebo
anthra(9,1,2-cde)benzo(rst)pentaphene-5,10-dione
Anthra[9,1,2-cde]benzo[rst]pentaphene-5,10-dione
Anthra[9.1.2-cde]benzo[rst]pentaphen-dichinon-(5.10.16.17)
Anthravat Dark Blue BO
anthravatdarkbluebo
Belanthrene Dark Blue BO
belanthrenedarkbluebo
Benzadone Dark Blue BOA
Benzadone Dark Blue BOR
Benzadone Dark Blue BORI | [EINECS(EC#)]
204-152-0 | [Molecular Formula]
C34H16O2 | [MDL Number]
MFCD00046330 | [Molecular Weight]
456.49 | [MOL File]
116-71-2.mol |
| Raw materials And Preparation Products | Back Directory | [Raw materials]
Potassium hydroxide-->Nitrogen-->Sodium acetate-->Sodium acetate trihydrate-->Sodium chlorate-->Naphthalene-->Benzanthrone-->Diffusing agent-->Triethylene glycol-->[4,4'-bi-7H-benz[de]anthracene]-7,7'-dione-->7H-Benz[de]anthracen-7-one, 2-chloro--->[3,3'-bi-7H-benz[de]anthracene]-7,7'-dione-->Anthra[9,1,2-cde]benzo[rst]pentaphene-->4,4'-dibenzoyl-1,1'-binaphtyl-->Vat Violet 10-->3-Bromobenzanthrone-->Anthracen-9-ol-->4,4'-dibenzoyl-1,1'-binaphtyl | [Preparation Products]
Vat Black 9-->INDANTHRENE BLACK BBN-->Vat Dark Blue VB-->vat black 16-->Anthra[9,1,2-cde]benzo[rst]pentaphene-5,10-dione, chloro derivs.-->INDANTHRENE BLACK BBN-->vat black 16 |
| Hazard Information | Back Directory | [General Description]
Bluish-black to black powder. | [Air & Water Reactions]
Insoluble in water. | [Fire Hazard]
Flash point data for this chemical are not available. VAT BLUE 20 is probably combustible. | [Description]
Violanthrone, Indanthren Dark
Blue BOA,is generated by
potash fusion of benzanthrone , .
Direct potash fusion leads to side products, such
as isoviolanthrone and 4-hydroxybenzanthrone.
The crude product may be used directly or in
mixtures with other dyes in vat dyeing. It is also
used to generate violanthrone derivatives. Several
processes have been developed to decrease
the amounts of undesirable side products of the
direct potash fusion of benzanthrone or to purify
the bibenzanthronyl or the crude violanthrone
. Especially effective is the addition
of high-boiling solvents such as naphthalene, the
so-called low carbazole anthracene residues, or
sodium acetate to the melt. The quality of the
products prepared by these methods is satisfactory
for the production of almost all violanthrone
dyes. | [Flammability and Explosibility]
Notclassified | [Synthesis]
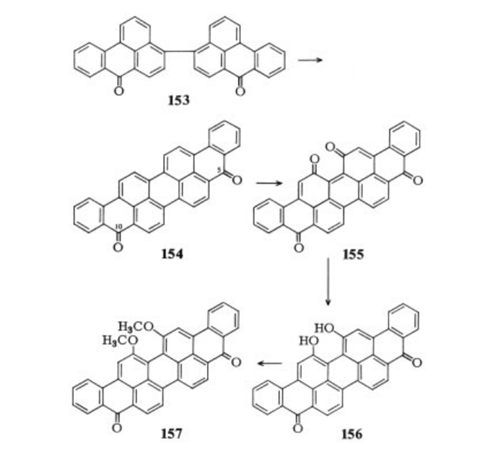
Violanthrone of high purity is obtained
from 4,4'-bibenzanthronyl (153) by alkaline or
acid ring closure, preferably in the presence
of oxidizing agents , . If this ring
closure is carried out in sulfuric acid with
an excess of manganese dioxide, the 16,17-
violanthronequinone (155) is obtained. This can
be reduced readily with sodium hydrogen sulfite
to 16,17-dihydroxyviolanthrone (156). Subsequent
alkylation of the hydroxy groups yields
very fast navy-blue to brilliant-green vat dyes.
The dimethyl ether (157) is the well-known
dye Caledon Jade Green or Indanthren Brilliant
Green B and FFB (extremely pure form).
 | [Properties and Applications]
|
TEST ITEMS
|
SPECIFICATION
|
|
APPEARANCE
|
BLUE POWDER
|
|
SHADE
|
REDDISH
|
|
HEAT RESISTANCE
|
300 °C min
|
|
LIGHT FASTNESS
|
8
|
|
ACID RESISTANCE
|
5
|
|
ALKALI RESISTANCE
|
5
|
|
FASTNESS TO BLEEDING
|
5
|
|
OIL ABSORPTION
|
40-50%
|
|
SPECIFIC SURFACE
|
27 m
2
/g
|
|
DENSITY
|
1.60 g/cm
3
|
|
RESIDUE ON 80 MESH
|
5.0% max
|
|
WATER SOLUBLE
|
1.0% max
|
|
VOLATITE 105 °C
|
1.0% max
|
|
TINTING STRENGTH
|
100-105 %
|
|
|
|