| Identification | More | [Name]
Levamisole | [CAS]
14769-73-4 | [Synonyms]
IMMUNOPURE(R) PHOSPHATASE SUPPRESSOR
L-6-PHENYL-2,3,5,6-TETRAHYDROIMIDAZOL(2,1-B) THIAZOLE
LEVAMISOLE
LEVAMISOLE BASE
(s)-2,3,5,6-tetrahydro-6-phenylimidazo[2,1-b]thiazole
Imidazo2,1-bthiazole, 2,3,5,6-tetrahydro-6-phenyl-, (6S)-
lepuron
levomysol
l-tetramisole
6-PHENYL-2,3,5,6-TETRAHYDROIMIDAZO[2,1-B]THIAZOLE (LEVAMISOLE)
(6S)-2,3,5,6-Tetrahydro-6-phenylimidazo[2,1-b]thiazole
Ketrax | [EINECS(EC#)]
238-836-5 | [Molecular Formula]
C11H12N2S | [MDL Number]
MFCD00792481 | [Molecular Weight]
204.29 | [MOL File]
14769-73-4.mol |
| Chemical Properties | Back Directory | [Melting point ]
60-61.5° | [alpha ]
D25 -85.1° (c = 10 in chloroform) | [Boiling point ]
344.4±45.0 °C(Predicted) | [density ]
1.32±0.1 g/cm3(Predicted) | [storage temp. ]
0-6°C | [solubility ]
Chloroform (Slightly), Methanol (Slightly) | [form ]
Solid | [pka]
10.00±0.40(Predicted) | [color ]
Off-White to Pale Yellow | [InChI]
InChI=1S/C11H12N2S/c1-2-4-9(5-3-1)10-8-13-6-7-14-11(13)12-10/h1-5,10H,6-8H2/t10-/m1/s1 | [InChIKey]
HLFSDGLLUJUHTE-SNVBAGLBSA-N | [SMILES]
S1CCN2C[C@H](C3=CC=CC=C3)N=C12 | [CAS DataBase Reference]
14769-73-4(CAS DataBase Reference) | [EPA Substance Registry System]
14769-73-4(EPA Substance) |
| Questions And Answer | Back Directory | [Pharmacology and mechanism of action]
Levamisole is the L-isomer of tetramisole and is more active than the racemic mixture. It was introduced in 1966 as a veterinary drug and a little later as a human anthelminthic drug against ascariasis. The drug has also shown to be effective against hookworms (Ancylostoma duodenale and Necator americanus), but results of reported studies are inconsistent [1]. The mechanism of action of levamisole in helminthiasis is through its stimulation of autonomic ganglia (nicotinic receptors) of the worms. On exposure to the drug, immature and adult worms show spastic contraction followed by tonic paralysis. This mechanism seems to be common to other anthelminthics such as pyrantel and bephenium hydroxynaphthoate [2]. In higher doses, levamisole acts as an immunostimulant. It restores depressed cell-mediated immune mechanisms in peripheral T-lymphocytes, but may have marginal effects in immunologically competent individuals [3]. The clinical implication of this effect in the treatment of helminthiasis is unknown.
| [Indications]
Monoinfections with Ascaris lumbricoides. In polyinfections, mebendazole is the drug of choice.
| [Side effects]
During the treatment of nematode infections the drug produces minor side effects including nausea, vomiting, abdominal pain and headache [4, 5]. During prolonged treatment as an immunomodulator in rheumatic arthritis and in cancer patients, serious side effects such as blood disorders (agranulocytosis, neutropenia and thrombocytopenia), kidney damage, influenza-like reactions, vasculitis, photosensitivity and allergy to the drug have been reported [6, 7].
| [Contraindications and precautions]
The drug should be avoided in patients allergic to the drug. Administration of levamisole may provoke a reaction similar to that seen after intake of alcohol together with disulfiram. During long-term treatment, patients with kidney damage or with blood disorders may experience exacerbation of their diseases.
| [Interactions]
Levamisole has been reported to displace the protein binding of rifampicin in vitro [8]. The clinical significance of this is as yet unknown.
| [References]
1. Miller MJ (1980). Use of levamisole in parasitic infections. Drugs, 19, 122–130.
2. van Wauwe J, Janssen PAJ (1991). On the biochemical mode of action of levamisole: an update. Int J Immunopharmacol, 13, 3–9.
3. Renoux G (1980). The general immunopharmacology of levamisole. Drugs, 19, 89–99.
4. Lionel ND, Mirando EH, Nanayakkara JC, Soysa PE (1969). Levamisole in the treatment of ascariasis in children. BMJ, 4, 340–341.
5. Farid Z, Bassily S, Miner WF, Hassan A, Laughli LW (1977). Comparative single-dose treatment of hookworm and roundworm infections with levamisole, pyrantel and bephenium. J Trop Med Hyg, 80, 107–108.
6. Chrisp P, McTavish D (1991). Levamisole/fluorouracil: A review of their pharmacology and adjuvant therapeutic use in colorectal cancer. Drugs & Aging, 14, 317–337.
7. Amery WK, Butterworth BS (1983) The dosage regimen of levamisole in cancer: is it related to efficacy and safety Int J Immunopharmacol, 5, 1–9.
8. Pérez-Gallardo L, Blanco ML, Soria H, Escanero JF (1992). Displacement of rifampicin bound to serum proteins by addition of levamisole. Biomed Pharmacother, 46, 173–174.
|
| Hazard Information | Back Directory | [Originator]
Solaskil,Specia,France,1971 | [Uses]
Biological response modifier. | [Uses]
Levamisole is used for initial and secondary immunodeficient conditions, autoimmune
diseases, chronic and reoccurring infections, large intestine adenocarcinoma, helmintosis,
and rheumatoid arthritis. Synonyms of this drug are decaris, tetramizole, and others.
Levimasole is also a drug of choice for ascardiasis. Numerous investigations show that a
single dose of levamisole heals from 90 to 100% of patients with ascardiasis, in particular
those infected with A. duodenale. It is less effective against ancylostomiasis and strongy�loidiasis. However, it is not effective against N. americanus. It seems likely that it has a
gangliostimulating effect on parasite tissues in both the parasympathetic and sympathetic
regions. Moreover, it is presumed that this drug has an immunomodulatory effect on the
host organism. Synonyms of this drug are decaris, solacil, ergamisol, tramisol, immunol,
and others. | [Definition]
ChEBI: A 6-phenyl-2,3,5,6-tetrahydroimidazo[2,1-b][1,3]thiazole that has S configuration. It is used (generally as the monohydrochloride salt) to treat parasitic worm infections in pigs, sheep and cattle and was formerly used in huma
s as an adjuvant to chemotherapy for the treatment of various cancers. It is also widely used as an adulterant to coccaine. | [Manufacturing Process]
To a stirred and refluxed suspension of 17 parts of 1,2-dibromoethane, 7.8
parts of sodium hydrogen carbonate and 50 parts of 2-propanol is added a
mixture of 3.4 parts of dl-2-thio-1-phenyl-imidazolidine, 9 parts of a 20%
potassium hydroxide solution in 40 parts of 2-propanol over a period of about
1 hour. After the addition is complete, the whole is stirred and refluxed for an
additional 3 hours. The reaction mixture is evaporated. To the residue are
added 18 parts of a 15% potassium hydroxide solution. The whole is extracted
with toluene. The extract is dried and evaporated. The oily residue is dissolved
in acetone and gaseous hydrogen chloride is introduced into the solution. The
precipitated solid salt is filtered off and recrystallized from 2-propanol, yielding
dl-2,3,5,6-tetrahydro-6-phenyl-imidazo[2,1-b]thiazole hydrochloride; melting
point 264°C to 266°C.
dl-6-phenyl-2,3,5,6-tetrahydroimidazo[2,1-b]thiazole hydrochloride, 188 g
(0.785 mol), is suspended in a mixture of 500 ml of water and 500 ml of
methylene chloride. The suspension is stirred mechanically while 20% sodium
hydroxide solution is added until the solution is basic. Ice is added from time
to time to keep the temperature below the boiling point of the methylene
chloride. The methylene chloride layer is separated, washed with water, dried
over potassium carbonate and evaporated. The oily residue crystallizes with
the evolution of the heat when poured into a beaker containing 100 ml of
ether. The free base is washed with ether. The yield of dl-6-phenyl-2,3,5,6-
tetrahydroimidazo[2,-b]thiazole is 151.4 g (0.746 mol), 94%. The product has
a melting point of 90°C.
A solution of 204.3 g (1 mol) of dl-6-phenyl-2,3,5,6-tetrahydroimidazo[2,1-
b]thiazole and 232.3 g (1 mol) of d-10-camphorsulfonic acid in 1,750 ml of
chloroform is allowed to crystallize overnight at -28°C. The solvate is
recovered by filtration and washed with ice cold chloroform (400 ml). The
solvate is dried (decomposed) under nitrogen 7 hours and then in air
overnight. The yield of d(+)6-phenyl-2,3,5,6-tetrahydroimidazo[2,1-b]thiazole
d-10-camphorsulfonate is 202.5 g (0.464 mol) 92.8%, melting point 139°C to
140°C [α]D25+ 82.6 (C = 16, H2O).
A solution of 150 g (0.344 mol) of d(+)6-phenyl-2,3,5,6-tetrahydroimidazo[2,1-b]thiazole, d-10-camphorsulfonate in water is treated with 15.5 g (0.378
mol) of 98% sodium hydroxide and the liberated base extracted with
chloroform. The chloroform solution is washed with water followed by sodium
chloride solution and dried over magnesium sulfate. Evaporation of the solvent
left 72.1 g of residue which crystallized shortly. The free base hereby obtained
has a melting point of 60°C to 61.5°C and an optical rotation [α]D25+ 85.1 (C
= 10, CHCl3).
The free base d(+)6-phenyl-2,3,5.6-tetrahydroimidazo[2.1-b]thiazole is
dissolved in 112 ml of acetone and 178 ml of isopropanolic hydrogen chloride
is added all at once. The hydrochloride crystallizes at once. After cooling to
below 0°C, the salt is recovered by filtration and washed with acetone. The
product weighs 75.2 g (0.312 mol), 91%, from the camphorsulfonate, melting
point 227°C to 227.5°C [α]D25+ 123.1 (C = 15, H2O). | [Brand name]
Ergamisol (Janssen). | [Therapeutic Function]
Antiinflammatory | [Antimicrobial activity]
Its principal activity is against Asc. lumbricoides and hookworms. Worms are paralyzed and passed
out in the feces within a few hours. | [Pharmaceutical Applications]
The l-isomer of tetramisole, available as the monohydrochloride.
The d-isomer has no anthelmintic activity. It is very soluble
in water and is stable in the dry state. | [Mechanism of action]
Levamisole has immunomodulating activity. It is believed that it regulates cellular mech�anisms of the immune system, and the mechanism of its action may be associated with
activation and proliferative growth of T-lymphocytes, increased numbers of monocytes and
activation of macrophages, and also with increased activity and hemotaxis of neutrophylic
granulocytes. Levamisole also exhibits anthelmint action. It also increases the body’s over�all resistivity and restores altered T-lymphocyte and phagocyte function. It can also fulfill
an immunomodulatory function by strengthening the weak reaction of cellular immunity,
weakening strong reaction, and having no effect on normal reaction. | [Pharmacokinetics]
Oral absorption: c. 90%
Cmax 150 mg oral: 0.5 mg/L after c. 2 h
Plasma half-life: c. 4 h
Volume of distribution: 100–120 L
Levamisole is rapidly absorbed from the gut and extensively
metabolized in the liver. It is excreted chiefly in the urine. | [Clinical Use]
Ascariasis
Hookworm infection
Levamisole has been used in rheumatoid arthritis and some
other conditions that are said to respond to its immunomodulatory
activity. | [Synthesis]
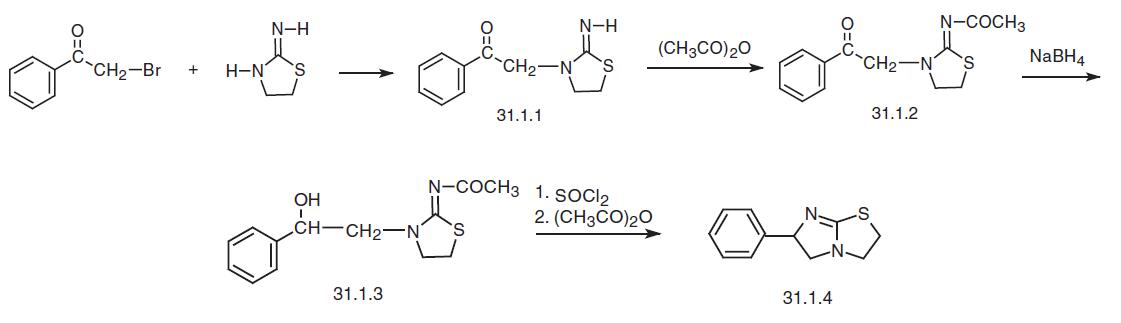
Levamisole, 2,3,5,6-tetrahydro-6-phenylimidazo[2,1-b]thiazole (31.1.4), is synthesized in various ways. One of them begins with α-bromoacetophenone, the reaction of which with 2-imino-1,3-thiazolidine gives 3-phenacyl-2-imino-1,3-thiazolidine (31.1.1). Reacting this product with acetic anhydride gives 3-phenacyl-2-acetylimino-1,3- thiazolidine (31.1.2). The ketone group in the resulting compound is reduced to an alcohol using sodium borohydride, and the resulting hydroxyl group in (31.1.3) is replaced with chlorine using thionyl chloride. Heating the product in acetic anhydride, the imidazole cycle closes, forming the product (31.1.4).
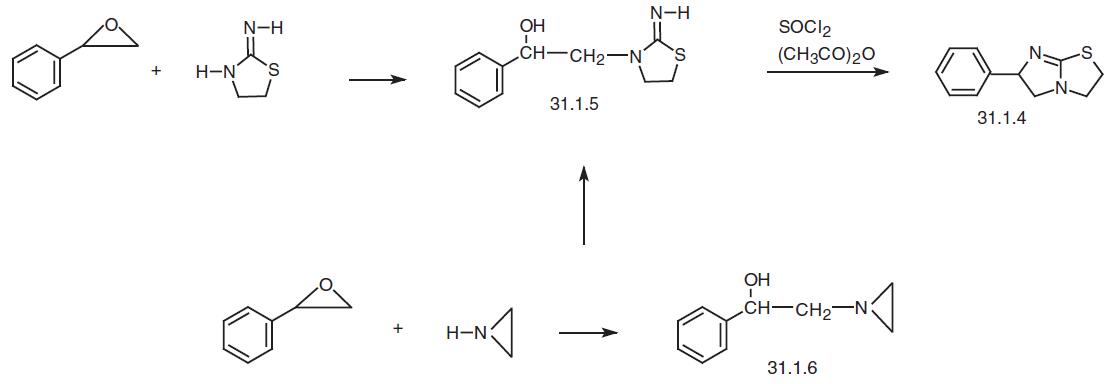
A somewhat different approach was realized when using styrene oxide as the initial start�ing material. Reacting it with 2-imino-1,3-thiazolidine gives 3-(2-phenyl-2-hydroxyethyl)- 2-imino-1,3-thiazolidine (31.1.5), which is subsequently treated with thionyl chloride and then acetic anhydride to give the desired levamisole (31.1.4).
Finally, the following scheme of making the product has been proposed using the same styrene oxide. Styrene oxide is reacted with aziridine, forming 2-aziridion-1- phenylethanol-1 (31.1.6). Treating this with potassium isothiocyanate or thiourea gives 3- (2-phenyl-2-hydroxyethyl)-2-amino-1,3-thiazolidine (31.1.5), and subsequent treatment with thionyl chloride (such as described above) and then with acetic anhydride gives the desired levamisole (31.1.4).
 | [Veterinary Drugs and Treatments]
Depending on the product licensed, levamisole is indicated for the
treatment of many nematodes in cattle, sheep and goats, swine,
poultry. In sheep and cattle, levamisole has relatively good activity
against abomasal nematodes, small intestinal nematodes (not
particularly good against Strongyloides spp.), large intestinal nematodes
(not Trichuris spp.), and lungworms. Adult forms of species
that are usually covered by levamisole, include: Haemonchus spp.,
Trichostrongylus spp., Osteragia spp., Cooperia spp., Nematodirus
spp., Bunostomum spp., Oesophagostomum spp., Chabertia spp., and
Dictyocaulus vivapurus. Levamisole is less effective against the immature
forms of these parasites, and is generally ineffective in cattle
(but not sheep) against arrested larval forms. Resistance of parasites
to levamisole is a growing concern.
In swine, levamisole is indicated for the treatment of Ascaris
suum, Oesophagostomum spp., Strongyloides, Stephanurus, and
Metastrongylus.
Levamisole has been used in dogs as a microfilaricide to treat
Dirofilaria immitis infection in the past, but is rarely used today.
It has also garnered some interest as an immunostimulant in the
adjunctive therapy of various neoplasms.
Because of its narrow margin for safety and limited efficacy
against many equine parasites, levamisole
is not generally used in
horses as an antiparasitic agent. It has been tried as an immune
stimulant, however. | [storage]
Store at -20°C |
|
|