| Identification | More | [Name]
1-(2,6-Dichlorophenyl)-2-indolinone | [CAS]
15307-86-5 | [Synonyms]
1-(2,6-DICHLOROPHENYL)-1,3-DIHYDRO-INDOLE-2-ONE
(N-1-(2,6-DICHLOROPHENYL)-2-INDOLIN-2-ONE
Diciofenac
2-[[2,6-Dichlorophenyl] amino] benzeneacetic acid
1-(2,6-DICHLOROPHENYL)-2-INDOLINONE /MEQUITAZINE
1-(2,6-DICHLOROPHENYL)-2-INDOLINONE/INDOLINONE
DICLOFENAC ACID/[2-(2,6-DICHLORO-PHENYLAMINO)-PHENY]-ACETIC ACID
(o-(2,6-dichloroanilino)phenyl)-acetic acid
dichlofenac
[2-(2,6-Dichloroanilino)phenyl]-acetic acid
2-[2-(2,6-Dichlorophenyl)aminophenyl]ethanoic acid
Diclofenac
DICLOFENAC FREE ACID
Rhumalgan
2-(2-(2,6-dichlorophenylamino)phenyl)acetic acid | [EINECS(EC#)]
239-348-5 | [Molecular Formula]
C14H9Cl2NO | [MDL Number]
MFCD00451393 | [Molecular Weight]
278.13 | [MOL File]
15307-86-5.mol |
| Questions And Answer | Back Directory | [Application in Particular Diseases]
In Osteoarthritis:
Topical diclofenac in a dimethyl sulfoxide carrier (Pennsaid) is a safe and effective treatment for Osteoarthritis pain. It is thought to act primarily by local inhibition of COX-2 enzymes.
|
| Hazard Information | Back Directory | [Originator]
Voltaren,Fujisawa,Japan,1974 | [Uses]
Diclofenac possesses all of the properties unique to the series of propionic acid drugs, yet
in terms of anti-inflammatory and analgesic strength it exceeds that of aspirin, analgin, and
ibuprofen. It is used in acute rheumatism, rheumatoid arthritis, osteoarthritis, ankylosing
spondylitis, arthrosis, back pain, neuralgia, and myalgia. It rarely causes side effects. The
most common synonym is voltaren. | [Uses]
prostaglandin synthetic inhibitor
| [Definition]
ChEBI: Diclofenac is a monocarboxylic acid consisting of phenylacetic acid having a (2,6-dichlorophenyl)amino group at the 2-position. It has a role as a non-narcotic analgesic, an antipyretic, an EC 1.14.99.1 (prostaglandin-endoperoxide synthase) inhibitor, a xenobiotic, an environmental contaminant, a drug allergen and a non-steroidal anti-inflammatory drug. It is a secondary amino compound, an amino acid, a dichlorobenzene, an aromatic amine and a monocarboxylic acid. It is functionally related to a phenylacetic acid and a diphenylamine. It is a conjugate acid of a diclofenac(1-). | [Indications]
Diclofenac (Voltaren, Cataflam) is approved for use
in rheumatoid arthritis, osteoarthritis, ankylosing
spondylitis, dysmenorrhea, and topically for the treatment treatment
of ocular inflammation and actinic keratosis.
Diclofenac exhibits approximately equal selectivity for
COX-1 and COX-2. The most common adverse reactions
are GI disturbances and headache.A reversible elevation
of serum transaminases occurs in 15% of patients. | [Manufacturing Process]
Four grams of N-chloroacetyl-N-phenyl-2,6-dichloroaniline and 4 grams of
aluminum chloride are well mixed together and heated for 2 hours at 160°C.
The melt is cooled and poured onto about 50 grams of ice while it is still
warm. The oil which separates is dissolved in 50 ml of chloroform, the
chloroform solution is washed with 10 ml of water, dried over sodium sulfate
and concentrated under 11 torr. The residue is distilled. The 1-(2,6-
dichlorophenyl)-2-indolinone melts at 126°-127°C.
A solution of 186 grams of 1-(2,6-dichlorophenyl)-2-indolinone in 660 ml of
ethanol and 660 ml of 2 N sodium hydroxide solution is refluxed for 4 hours.
The solution is then cooled and left to stand for 4 hours at 0°-5°C. The
crystals which form are filtered off and recrystallized from water. The sodium
salt of 2-(2,6-dichloroanilino)-phenylacetic acid melts at 283°-285°C. The
yield is 97% of theoretical, according to US Patent 3,558,690. | [Therapeutic Function]
Antiinflammatory | [Biological Functions]
Diclofenac (Voltaren) is a phenylacetic acid derivative
that is a potent inhibitor of COX and that has analgesic,
antiinflammatory, and antipyretic effects. Its use is accompanied
by side effects similar to those of other
NSAIDs. Indications for the drug include rheumatoid
arthritis, osteoarthritis, and ophthalmic inflammation
(use of an ophthalmic preparation). | [Biological Activity]
Diclofenac is an orally availablepotent and selective nonsteroidal anti-inflammatory drug (NSAID). Diclofenac is used to tre at pain and inflammatory diseases. It inhibits both cycloxygenase-1 (COX-1) and cycloxygenase-2 (COX-2). | [Mechanism of action]
Diclofenac is unique among the NSAIDs in that it possesses three possible mechanisms
of action: 1) inhibition of the arachidonic acid cyclooxygenase system (3 to 1,000 times more potent than other
NSAIDs on a molar basis), resulting in a decreased production of prostaglandins and thromboxanes; 2)
inhibition of the lipoxygenase pathway, resulting in decreased production of leukotrienes, particularly the
pro-inflammatory LKB4; and 3) inhibition of arachidonic acid release and stimulation of its reuptake, resulting in a
reduction of arachidonic acid availability. | [Pharmacokinetics]
Diclofenac is rapidly and completely (~100%) absorbed on oral administration, with peak plasma levels being
reached within 1.5 to 2.5 hours. The free acid (pKa = 4.0) is highly bound to serum proteins (99.5%), primarily
albumin. Only 50 to 60% of an oral dose is bioavailable because of extensive hepatic metabolism. | [Clinical Use]
Diclofenac is synthesized from N-phenyl-2,6-dichloroaniline. It is available in 120 different countries and,
perhaps, is the most widely used NSAID in the world. It was introduced in the United States in 1989 but was first
marketed in Japan in 1974. It ranks among the top prescription drugs in the United States. Diclofenac possesses
structural characteristics of both arylalkanoic acid and the anthranilic acid classes of anti-inflammatory drugs, and it
displays anti-inflammatory, analgetic, and antipyretic properties. | [Synthesis]
Diclofenac, 2-[(2,6-dichlorophenyl)-amino]-phenylacetic acid (3.2.42), is
synthesized from 2-chlorobenzoic acid and 2,6-dichloroaniline. The reaction of these in
the presence of sodium hydroxide and copper gives N-(2,6-dichlorophenyl)anthranylic
acid (3.2.38), the carboxylic group of which undergoes reduction by lithium aluminum
hydride. The resulting 2-[(2,6-dicholorphenyl)-amino]-benzyl alcohol (3.2.39) undergoes
further chlorination by thionyl chloride into 2-[(2,6-dichlorophenyl)-amino]-ben�zylchloride (3.2.40) and further, upon reaction with sodium cyanide converts into 2-[(2,6-dicholorophenyl)-amino]benzyl cyanide (3.2.41). Hydrolysis of the nitrile group
leads to diclofenac (3.2.42) [107,108].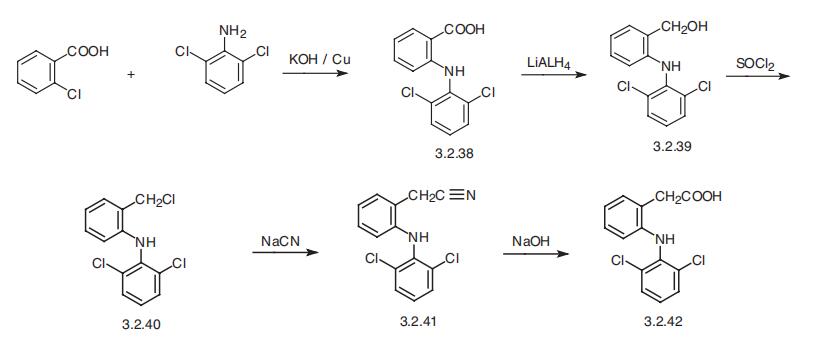
| [Metabolism]
Four major
metabolites resulting from aromatic hydroxylation have been identified. The major metabolite via CYP3A4 is the
4′-hydroxy derivative and accounts for 20 to 30% of the dose excreted, whereas the 5-hydroxy, 3′-hydroxy, and
4′,5-dihydroxy metabolites via CYP2C9 account for 10 to 20% of the excreted dose. The remaining drug is excreted
in the form of sulfate conjugates. Although the major metabolite is much less active than the parent compound, it may
exhibit significant biological activity, because it accounts for 30 to 40% of all of the metabolic products. |
|
|