| Identification | More | [Name]
Posaconazole | [CAS]
171228-49-2 | [Synonyms]
Posaconazole
2,5-Anhydro-1,3,4-trideoxy-2-C-(2,4-difluorophenyl)-4-[[4-[4-[4-[1-[(1S,2S)-1-ethyl-2-hydroxypropyl]-1,5-dihydro-5-oxo-4H-1,2,4-triazol-4-yl]phenyl]-1-piperazinyl]phenoxy]methyl]-1-(1H-1,2,4-triazol-1-yl)-D-threo-pentitol
Noxafil
Sch 56592
4-[4-[4-[4-[[(3R,5R)-5-(2,4-Difluorophenyl)-5-(1,2,4-triazol-1-ylmethyl)oxolan-3-yl]methoxy]phenyl]piperazin-1-yl]phenyl]-2-[(2S,3S)-2-hydroxypentan-3-yl]-1,2,4-triazol-3-one
HYDROXYPROPYL]-2,4-DIHYDRO-3H-1,2,4-TRIAZOL-3-ONE
Posaconazole for research | [EINECS(EC#)]
682-747-8 | [Molecular Formula]
C37H42F2N8O4 | [MDL Number]
MFCD00941162 | [Molecular Weight]
700.78 | [MOL File]
171228-49-2.mol |
| Chemical Properties | Back Directory | [Appearance]
White Solid | [Melting point ]
170-1720C | [Boiling point ]
850.7±75.0 °C(Predicted) | [density ]
1.36±0.1 g/cm3(Predicted) | [Fp ]
9℃ | [storage temp. ]
-20°C | [solubility ]
Chloroform (Slightly), Methanol (Slightly) | [form ]
powder | [pka]
14.72±0.20(Predicted) | [color ]
white to beige | [optical activity]
[α]/D -24 to -32°, c = 1.0 in chloroform-d | [Usage]
Orally active triazole antifungal. | [Merck ]
14,7602 | [InChIKey]
RAGOYPUPXAKGKH-XAKZXMRKSA-N | [SMILES]
[C@]1(OC[C@@H](COC2C=CC(N3CCN(C4=CC=C(N5C=NN([C@@H](CC)[C@@H](O)C)C5=O)C=C4)CC3)=CC=2)C1)(CN1N=CN=C1)C1=CC=C(F)C=C1F |&1:0,3,22,25,r| | [CAS DataBase Reference]
171228-49-2(CAS DataBase Reference) |
| Hazard Information | Back Directory | [Description]
Posaconazole, launched in the UK, is the newest member of the azole
class of antifungal agents to reach the market. It is indicated for the treatment and
prophylaxis of a range of invasive fungal infections, including aspergillosis,fusariosis, chromoblastomycosis, mycetoma, and coccidiomycosis in patients
who are refractory to, or intolerant of, standard therapy with amphotericin B
and/or itraconazole. In the US, it is approved for the prophylaxis of invasive
Aspergillus and Candida infections in patients 13 years of age who are at high
risk of developing these infections due to being severely immunocompromised.
Additionally, it is approved for the treatment of oropharyngeal candidiasis.
Posaconazole has an expanded spectrum of activity over other members of the
azole antifungals. In addition to potent activity against refractory cases of
aspergillosis and fluconazole-resistant Candida, it demonstrates activity against
Zygomycetes. | [Chemical Properties]
White Solid | [Originator]
Schering-Plough (US) | [Uses]
Orally active triazole antifungal. | [Uses]
Posaconazole is a sterol C14ɑ demethylase inhibitor with an IC50 of 0.25 nM | [Definition]
ChEBI: An N-arylpiperazine that consists of piperazine carrying two 4-substituted phenyl groups at positions 1 and 4. A triazole antifungal drug. | [Brand name]
Noxafil | [Antimicrobial activity]
The spectrum includes dimorphic fungi (Blast. dermatitidis,
Coccidioides spp., Hist. capsulatum, Pen. marneffei, and Spor.
schenckii), molds (Aspergillus spp., Mucor spp., Rhizomucor
spp. and Rhizopus spp.), some dematiaceous fungi and yeasts
(Candida spp. and Cryptococcus spp.). | [General Description]
Technetium (99mTc) exametazime is a mixture of unstablelipophilic enantiomers that rapidly cross the blood-brain barrierand is trapped in the tissues. The proposed trapping mechanismfor localization includes reduction by glutathione. Asimilar diffusion and trapping process occurs with autologouslymphocytes in vitro.
Exametazime is also known as hexamethylpropyleneamineoxime or HMPAO. The radiolabeled complex is indicated for cerebral perfusion in stroke, but is most commonlyused for the radiolabeling of autologous leukocytesas an adjunct in the localization of intra-abdominal infectionand inflammatory bowel disease.
Each kit includes several components: (a) reaction vialscontaining a mixture of exametazime, stannous chloride,and sodium chloride; (b) vials of 1% methylene blue; (c)vials of phosphate buffer in 0.9% NaCl; and (d) 0.45-μm syringefilters. Product preparation depends on the intendeduse. | [Pharmaceutical Applications]
A synthetic triazole available for oral administration. | [Biochem/physiol Actions]
Posaconazole is a highly potent broadspectrum antifungal agent against the yeast infection caused especially by Candida sp. It blocks the growth of fungi by inhibiting the enzyme?lanosterol?14α-demethylase (CYP51). In contrast to other antifungal azoles, posaconazole has been reported not to induce the efflux pump mechanism. Posaconazole exhibits antichagasic effects against different strains of Trypanosoma cruzi causing Chagas disease. | [Clinical Use]
Invasive aspergillosis
Fusarium infection
Chromoblastomycosis and mycetoma
Coccidioidomycosis
Oropharyngeal candidosis
Prophylaxis of invasive fungal infections in patients at serious risk
With the exception of oropharyngeal candidosis and prophylaxis,
use is presently restricted to patients with disease that
is refractory to other antifungal drugs, or who are intolerant
to them. | [Side effects]
It is generally well tolerated even for long periods. Unwanted
effects include gastrointestinal discomfort and mild to moderate,
transient abnormalities of liver enzymes. Rare side effects
include cholestasis and hepatic failure. | [Synthesis]
Several routes to the synthesis
of posaconazole have been published in the literature. The most likely route to large scale synthesis uses
convergent synthesis of a key chiral THF subunit 101 and
aryl piperazine amine 102 followed by introduction of the
triazole subunit at the end.The readily
accessible allyl alcohol 94 was brominated
(PBr3) to give bromide 95 which was alkylated with sodium
diethylmalonate and the resulting diester was reduced with
NaBH4/LiCl, to give the key diol 97 in very good yields.
After scanning many hydrolases to desymmetrize the diol via
selective acylation, hydrolase SP 435 was found to be suitable. Thus reaction of the diol 97 in the presence of SP
435 with vinyl acetate in acetonitrile gave monoacetate 98 in
greater than 90% yield. Iodine mediated cyclization of the
monoacetate 98 with iodine in dichloromethane gave chiral
iodide 99 in 86% yield. The iodide was converted to triazole
(sodiumtriazole, DMF: DMPU) and immediately followed
by hydrolysis of the acetate with sodium hydroxide to provide
alcohol 100. Activation of the alcohol to the pchlorobenzene
sulfonate 101 proceeded in 76% yield which
was then coupled with commercially available amino alcohol
piperazine 102 with aqueous sodium hydroxide in DMSO to
give amine intermediate 103 in 96% yield. The amine was reacted with benzoyl chloride to give benzoate 104 (97%),
which was subsequently converted to triazine of posaconazole.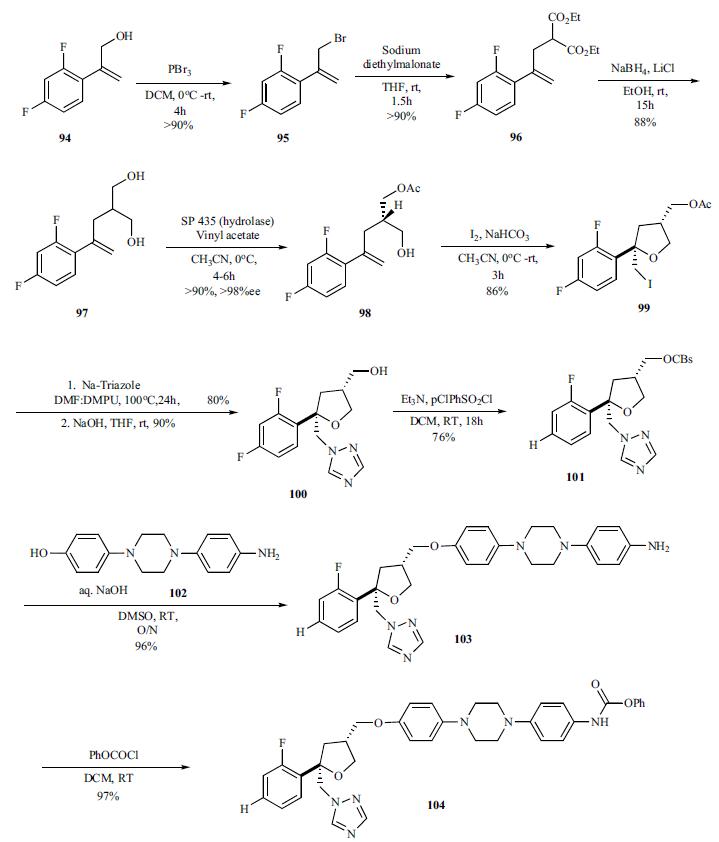
For the preparation of chiral hydrazine 107, intermediate
needed to make the triazolone, lactam 105 was reduced with
Red-Al to give (S)-2-benzyloxy propanal 106 (94%) which
was then reacted with formyl hydrazine to give hydrazone
107 in 81% yield. Addition of EtMgBr directly to formyl
hydrozones 107 gave mixture of (S,S)stereoisomer 109 and
(S,R)-diastereomer 110 in relative good diastereoselectivity
(94:6) in 55% yield. However, protection of the formyl
group as TBDMS ether 108 followed by treatment of the
EtMgCl gave 95% yield of the (S,S)-diastereomer 109 and
(S,R)-diastereomer 110 in 99:1 ratio.
For finishing off the synthesis, the formyl hydrazine 109
was coupled with the phenyl carbamate 104 in toluene at 75
- 85°C for 12 – 24 hrs. After the completion of coupling, the
intermediate was heated at 100 – 110°C for 24 – 48 hrs to
completely cyclize to the benzyloxy triazolone 108, which
was deprotected with 5% Pd/C and formic acid at room temperature
overnight and 40°C for 24 h to give posaconazole
(XV) in 80% overall yield. 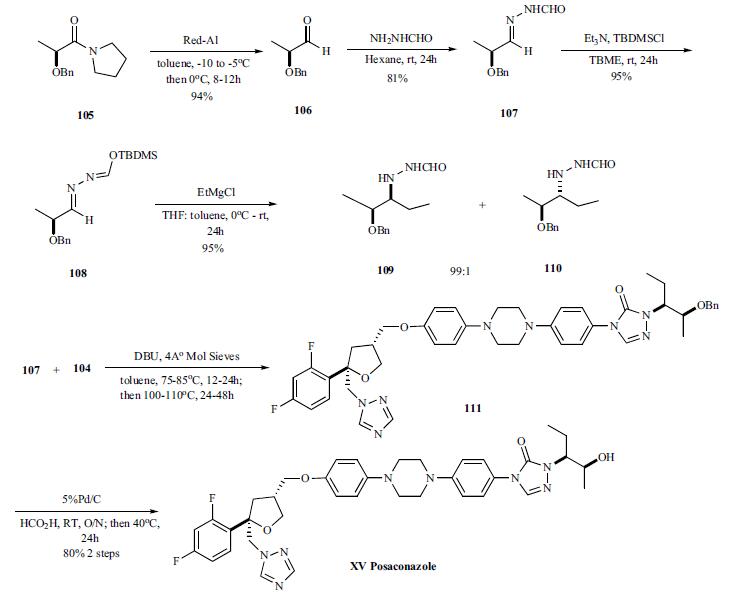 | [target]
C14ɑ demethylase | [Drug interactions]
Potentially hazardous interactions with other drugs
Analgesics: concentration of fentanyl possibly
increased.
Anti-arrhythmics: avoid concomitant use with
dronedarone.
Antibacterials: rifamycins may reduce posaconazole
concentration; avoid unless benefit outweighs risk;
rifabutin concentration increased.
Anticoagulants: avoid with apixiban and rivaroxaban.
Antidepressants: avoid concomitant use with
reboxetine.
Antidiabetics: posaconazole can decrease glucose
concentrations, monitor glucose levels in diabetic
patients; possibly enhances hypoglycaemic effect of
glipizide.
Antiepileptics: phenytoin, fosphenytoin,
carbamazepine, phenobarbital and primidone may
reduce posaconazole concentration - avoid unless
benefit outweighs risk.
Antimalarials: avoid with artemether/lumefantrine
and piperaquine with artenimol.
Antipsychotics: increased risk of ventricular
arrhythmias with pimozide - avoid; possibly increase
quetiapine levels - reduce dose of quetiapine;
possibly increases lurasidone concentration - avoid.
Antivirals: concentration of atazanavir increased and
possibly daclatasvir and simeprevir (reduce dose of
daclatasvir, avoid with simeprevir); concentration
reduced by efavirenz and possibly fosamprenavir;
possibly increases saquinavir levels; increased
risk of ventricular arrhythmias with telaprevir;
concentration of both drugs increased with dasabuvir
and paritaprevir - avoid.
Anxiolytics and hypnotics: increases midazolam
levels.
Ciclosporin: increases posaconazole concentration;
posaconazole can increase ciclosporin concentration
- dose reduction may be required.
Cytotoxics: concentration of bosutinib increased
- avoid or reduce dose of bosutinib; possibly
increased everolimus concentration - avoid;
avoid with lapatinib; reduce dose of panobinostat
and ruxolitinib; possibly inhibits metabolism
of vinblastine and vincristine, increased risk of
neurotoxicity.
Ergot alkaloids: may increase ergot alkaloid
concentration leading to ergotism - avoid.
Guanfacine: possibly increases guanfacine
concentration - halve guanfacine dose.
Ivacaftor: possibly increased concentration of
ivacaftor.
Lipid-lowering drugs: avoid with lomitapide;
possibly increased risk of myopathy with atorvastatin
or simvastatin - avoid.1
Lumacaftor: posaconazole concentration possibly
reduced - reduce dose of lumacaftor with ivacaftor.
Ranolazine: possibly increased ranolazine
concentration - avoid.
Sirolimus: may increase concentration of sirolimus -
adjust sirolimus dose as required according to levels.
Sulphonylureas: posaconazole can decrease glucose
concentrations, monitor glucose levels in diabetic
patients.
Tacrolimus: increases Cmax and AUC of tacrolimus
by 121% and 358% respectively - reduce tacrolimus
dose to about a third of current dose and adjust as
required.
Ulcer-healing drugs: cimetidine may reduce
posaconazole concentration by 39% - avoid unless
benefit outweighs risk; avoid with histamine H2-
antagonists and proton pump inhibitors. | [Metabolism]
Limited metabolism, most circulating metabolites are
glucuronide conjugates with only small amounts of
oxidative metabolites. The main elimination route of
posaconazole is via the faeces (77%) where 66% of a dose
is excreted unchanged. About 14% of a dose is excreted in
the urine with only trace amounts excreted unchanged. | [References]
[1] hanan k. munayyer, paul a. mann, andrew s. chau, taisa yarosh-tomaine, jonathan r. greene, roberta s. hare, larry heimark, robert e. palermo, david loebenberg and paul m. mcnicholas. posaconazole is a potent inhibitor of sterol 14α-demethylation in yeasts and molds. antimicrobial agents and chemotherapy. 2004; 48(10): 3690-3696
[2] daryl s. schiller and horatio b. fung. posaconazole: an extended-spectrum triazole antifungal agent. clinical therapeutics. 2007; 29(9): 1862-1886 |
| Questions And Answer | Back Directory | [Pharmacological effects]
Posaconazole (posaconazole) is derived from itraconazole. It is currently subject to III phase clinical trials. Its pharmacological effects are similar with azoles, but compared with itraconazole, it has a stronger inhibitory effect on the C14 demethylation of steroid, especially for Aspergillus.
| [Pharmacokinetics]
Studies on dosage and dosage protocol have shown that the rate of absorption and elimination rate is in line with the single-compartment model. There are significant differences on the relative bioavailability of different doses of oral suspension. It can be taken separately (every 12 hours or every 6 hours) which can significantly improve the bioavailability with the protein binding rate of 98% to 99%. With respect to tablets, the bioavailability of suspensions increase and food can significantly improve the speed and extent of absorption of drug absorption. An investigation of renal dysfunction on the pharmacokinetics of the drug study results has showed that the drug can’t be removed by hemodialysis without being affected by hemodialysis. Single-dose study has showed that patients with varying degrees of chronic kidney disease have no necessity for dosage adjustment. The Half-life of this is about 25 hours which can be primarily metabolized by the liver.
| [Clinical indications and usage]
It can be clinically used for the treatment of aspergillosis, zygnmycosis, and fusariumsis and can also be used for infection caused by part of fluconazole-resistant Candida genus. Studies have shown that posaconazole can widely and effectively applied to the treatment of phaeohyphomycosis and improve the infection survival rate of dermatitidis infection in a dose-dependent manner. The drug, as second-line drugs, has an effective rate of 44% to 78% against the invasive aspergillosis which is resistant to amphotericin B and itraconazole. It also has an effective rate of 71% against the zygomycete fungi. The drug is an oral suspension with the recommended dose of 200mg and 4 times per day with meals and taken orally for 7 to 10 days. This dose can also be maintained or changed to 400mg with oral administration of 2 times per day. The steady-state plasma concentration can reach within 7 to 10 days.
The above information is edited by the Chemicalbook of Dai Xiongfeng.
|
|
|