| Identification | More | [Name]
DIISOPROPYLCHLOROSILANE | [CAS]
2227-29-4 | [Synonyms]
CHLORODIISOPROPYLSILANE
DIISOPROPYLCHLOROSILANE
Silane, chlorobis(1-methylethyl)-
Diisopropylsilyl chloride | [Molecular Formula]
C6H15ClSi | [MDL Number]
MFCD00054896 | [Molecular Weight]
150.72 | [MOL File]
2227-29-4.mol |
| Chemical Properties | Back Directory | [Boiling point ]
58-60 °C50 mm Hg(lit.) | [density ]
0.883 g/mL at 25 °C(lit.)
| [refractive index ]
n20/D 1.429(lit.)
| [Fp ]
72 °F
| [storage temp. ]
Inert atmosphere,Room Temperature | [solubility ]
generally sol organic solvents; reacts with alcohols,
ammonia,and water. | [form ]
clear liquid | [color ]
Colorless to Almost colorless | [Specific Gravity]
0.883 | [Hydrolytic Sensitivity]
8: reacts rapidly with moisture, water, protic solvents | [BRN ]
2231355 | [InChI]
InChI=1S/C6H15ClSi/c1-5(2)8(7)6(3)4/h5-6,8H,1-4H3 | [InChIKey]
CGXYLRTYLIHXEE-UHFFFAOYSA-N | [SMILES]
[SiH](Cl)(C(C)C)C(C)C | [CAS DataBase Reference]
2227-29-4(CAS DataBase Reference) | [EPA Substance Registry System]
Silane, chlorobis(1-methylethyl)- (2227-29-4) |
| Safety Data | Back Directory | [Hazard Codes ]
C | [Risk Statements ]
R10:Flammable.
R34:Causes burns. | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection .
S45:In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) . | [RIDADR ]
UN 2986 8/PG 2
| [WGK Germany ]
3
| [F ]
10-21 | [TSCA ]
Yes | [HazardClass ]
3.1 | [PackingGroup ]
II | [HS Code ]
29319090 |
| Hazard Information | Back Directory | [Physical properties]
bp 150–153°C,bp 54–55°C/45 mmHg;
d 0.872 gmL?1 | [Uses]
Intramolecular
hydrosilylation is also possible within β-diisopropylsilyloxy
esters (13), constituting an exceptionally mild method for reducing
ester groups to the aldehyde oxidation level (eq 4).The derivatives
(13) may be synthesized from β-hydroxy esters (12) as described
above for the analogous ketones. Treatment with fluoride
ions (but not Lewis acids) induces hydride transfer to give
alkoxysiladioxanes (14) in excellent yields (≥95%). Although
usually performed in dichloromethane, the hydrosilylation may
also be accomplished with ethyl acetate as solvent, providing
strong evidence for intramolecularity.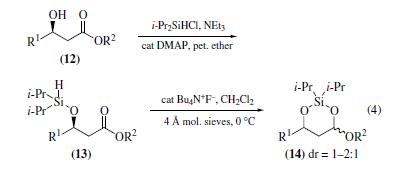
| [Application]
Used in a silylation-reduction-allylation sequence of
β-hydroxy esters to homoallylic-substituted 1,3-diols.
Used in the silylation-hydrosilation-oxidation of allyl
alcohols to 1,3-diols. Reaction carried out in
diastereoselective manner. Reduces β-hydroxy ketones to
anti-1,3 diols. | [Preparation]
obtained by reaction of trichlorosilane with
isopropylmagnesium chloride;the original yield of 45% may
be raised to 70–80% by employing conc hydrochloric acid to
quench the reaction. | [Purification Methods]
Impurities can be readily detected by 1H NMR. Purify it by fractional distillation [Gilman & Clark J Am Chem Soc 69 1499 1947, Allen et al. J Chem Soc 3668 1957]. |
|
|