| Identification | More | [Name]
Cefapirin sodium | [CAS]
24356-60-3 | [Synonyms]
CEFADYL
CEPHAPIRIN
CEPHAPIRIN BENZATHINE
CEPHAPIRIN SODIUM
CEPHAPIRIN SODIUM SALT
2,0)oct-2-ene-2-carboxylicacid,3-(hydroxymethyl)-8-oxo-5-thia-1-azabicyclo(
7-(2-(4-pyridylthio)acetamido)-,acetate(ester),monosodiumsalt
blp1322
cefaloject
cefapirinsodium
cefaprinsodium
cefatrexyl
cephatrexil
cephatrexyl
sodiumcefapirin
sodiumcephapirin
cephapirin sodium crystalline
sodium (6R-trans)-3-(acetoxymethyl)-8-oxo-7-[(4-pyridylthio)acetamido]-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylate
CEPHAPIRIN BEUZATHINE
5-Thia-1-azabicyclo4.2.0oct-2-ene-2-carboxylic acid, 3-(acetyloxy)methyl-8-oxo-7-(4-pyridinylthio)acetylamino-, monosodium salt, (6R,7R)- | [EINECS(EC#)]
246-194-2 | [Molecular Formula]
C17H17N3O6S2 | [MDL Number]
MFCD00864868 | [Molecular Weight]
423.46 | [MOL File]
24356-60-3.mol |
| Chemical Properties | Back Directory | [Appearance]
White or pale yellow powder. | [Melting point ]
>177°C (dec.) | [alpha ]
+152~+170゜(25℃/D)(c=2,H2O)(calculated on the dehydrous basis) | [storage temp. ]
0-6°C | [solubility ]
Soluble in water, practically insoluble in methylene chloride. | [form ]
powder | [pka]
pKa 2.15 (Uncertain) | [color ]
Light Beige to Beige | [InChIKey]
VGEOUKPOQQEQSX-OALZAMAHSA-M | [CAS DataBase Reference]
24356-60-3(CAS DataBase Reference) |
| Safety Data | Back Directory | [Hazard Codes ]
Xn | [Risk Statements ]
R36/37/38:Irritating to eyes, respiratory system and skin .
R42/43:May cause sensitization by inhalation and skin contact . | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36:Wear suitable protective clothing . | [RTECS ]
XI0382000 | [HS Code ]
2941906000 |
| Hazard Information | Back Directory | [Description]
Cephapirin has a pyridylthiomethylene containing side chain at C-7. It is comparatively resistant to
staphylococcal β-lactamase, although it is sensitive to many other β-lactamases. Cephapirin also is sensitive
to host deacetylation in the liver, kidneys, and plasma, which reduces potency by about half. Nonetheless, it
finds significant use in the parenteral treatment of infections because of susceptible bacteria. It is a
substitute for the nafcillin subgroup of penicillins. It is not orally active. It is comparatively painful on IM
injection, and its doses must be reduced in the presence of renal impairment. Following injection, it is
excreted primarily in the urine, partly by glomerular filtration and partly by tubular secretion. | [Chemical Properties]
White or pale yellow powder. | [Originator]
Cefadyl,Bristol,US,1974 | [Uses]
Cephalosporin antibacterial. | [Application]
Cephapirin was synthesized by BristolMyers Laboratories in 1970. It shows almost the same in vitro antibacterial activity as cephalothin, but its in vivo effects are slightly greater than those of cephalothin. Like cephalothin, it is metabolized in vivo, and its deacetylated metabolite shows almost the same activity against gram-positive bacteria as cephalothin, but weaker activity against gramnegative bacteria. Cephapirin has been used for therapy of urinary tract infections and osteomyelitis caused by Staphylococcus, Streptococcus, and Escherichia coli. | [Definition]
ChEBI: The sodium salt of cephapirin. A first-generation cephalosporin antibiotic, it is effective against gram-negative and gram-positive organisms. Being more resistant to beta-lactamases than penicillins, it is effective agains most staphyloco
ci, though not methicillin-resistant staphylococci. | [Manufacturing Process]
One route is that described in US Patent 3,422,100 as follows, starting with
aminocephalosporanic acid (ACA): 27.2 g (0.1 mol) of 7-ACA, 33.2 g (0.3
mol) of NaHCO3, 200 ml of water and 100 ml of acetone were mixed together,
cooled to 0°C and stirred rapidly while 20.1 g (0.1 mol) of bromoacetyl
bromide dissolved in 100 ml of acetone was added in one fast addition. The
temperature was kept at 0 to 5°C for ten minutes, then the ice-salt bath was
removed and stirring continued for one hour as the temperature approached
25°C. The mixture was concentrated in vacuo at 20°C to one-half volume and
200 ml of water added. Two 400 ml ether extracts were made and discarded.
The aqueous solution was covered with 200 ml of ethyl acetate and vigorously
stirred and cooled while being acidified to pH 2 with 40% phosphoric acid.
The mixture was filtered, the ethyl acetate layer separated and washed with
three 100 ml portions of water, dried over Na2SO4, filtered and treated with
30 ml of sodium 2-ethylhexanoate in n-butanol (34 ml = 0.1 mol). The oil
which settled out was scratched to induce crystallization. After stirring for 20 minutes the product, sodium 7-(α-bromoacetamido)cephalosporanate, was
scraped from the sides of the flask and collected. The filter cake was washed
with several portions of acetone, air dried, and dried in vacuo over P2O5.The
yield was 22.5 g and decomposed at 193°C.
A solution of 1.13 g (0.01 mol) of 2-mercaptopyrimidine and 1.06 g (0.01
mol) of sodium carbonate dissolved in 25 ml of water was added dropwise
over a period of an hour at room temperature, to a stirred solution of 4.15 g
(0.01 mol) of sodium 7-(α-bromoacetamido)cephalosporanate in 25 ml of
water.
Stirring was continued an additional 90 minutes and then 50 ml of ethyl
acetate was added, Forty percent H3PO4 was added dropwise with vigorous
stirring until pH 2.5 to 3 was obtained. The product crystallized immediately
and was filtered off, washed several times with water and then three times
with 25 ml portions of ethyl acetate, following which it was air dried. The yield
was 2.9 g of crystals that decomposed at 167 to 168°C. The IR and NMR
spectra were consistent with the desired product, 7-[α-(2-pyrimidinylthio)
acetamido]-cephalosporanic acid monohydrate.
An alternate route is that described in US Patent 3,503,967 which uses ACA in
the last step.
Another alternative route is that described in US Patent 3,578,661 uses
bromomethylcephalosporin as one raw material.
However the acid is prepared, the sodium salt may be prepared as described
in US Patent 3,503,967: Five liters of methylene chloride were added to a
clean dry vessel equipped with stirrer. 7-[α(4-pyridylthio)acetamido]
cephalosporanic acid (1,000 g) was added to the vessel, followed by 350 ml of
triethylamine. The resultant solution was treated with decolorizing charcoal for
15 minutes and filtered. A solution of sodium-3-ethyl-hexanoate (27.3%) in
butanol-methylene chloride was added to the filtrate with stirring. Seven
thousand five hundred milliliters of acetone was added. Crystallization
occurred while stirring was continued several hours under dry conditions. The
crystals were collected by filtration, washed with large volumes of acetone,
and then dried in vacuo at 50°C to yield about 950 g of the title compound. | [Brand name]
Cefadyl (Apothecon). | [Therapeutic Function]
Antibacterial | [Clinical Use]
Cephapirin (Cefadyl) is a semisynthetic 7-ACA derivativereleased in the United States in 1974. It closely resemblescephalothin in chemical and pharmacokinetic properties. Likecephalothin, cephapirin is unstable in acid and must beadministered parenterally in the form of an aqueous solutionof the sodium salt. It is moderately protein bound (45%–50%)in plasma and cleared rapidly by the kidneys. Cephapirin andcephalothin are very similar in antimicrobial spectrum andpotency. Conflicting reports concerning the relative occurrenceof pain at the site of injection and thrombophlebitis afterintravenous injection of cephapirin and cephalothin are difficultto assess on the basis of available clinical data. | [Synthesis]
Cephapirin, (6R-trans)-3-[(acetyloxy)methyl]-8-oxo-7-[[(4-pyridinylthio)
acetyl]amino]-5-thia-1-azabicyclo[4.2.0]oct-2-en-2-carboxylic acid (32.1.2.4), is synthe�sized by acylating 7-aminocephalosporanic acid with 4-pyridylthioacetic acid chloride
(32.1.2.3), which is synthesized by reacting 4-chloropyridine with mercaptoacetic acid in
the presence of a base, forming 4-pyridylthioacetic acid (32.1.22), and further transform�ing the resulting acid to the acid chloride by reacting it with phosphorous pentachloride.
An alternative way of making cephapirin is the acylation of 7-aminocephalosporanic acid
by bromoacetyl bromide, which gives a bromoacetyl derivative (32.1.2.5), and which is
then reacted with 4-mercaptopyridine in the presence of triethylamine, forming the desired
cephapirin (32.1.2.4).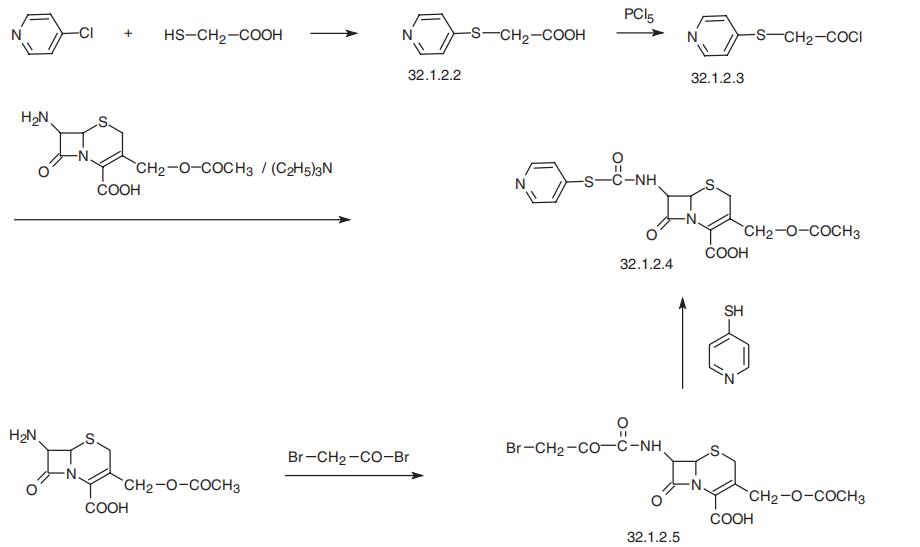
| [storage]
4°C, protect from light |
|
|