| Identification | More | [Name]
1-(Diphenylmethyl)-3-azetidinyl methanesulfonate | [CAS]
33301-41-6 | [Synonyms]
1-BENZHYDRYL-3-METHANESULFONATOAZETIDINE
1-BENZHYDRYL-3-METHANESULFONYLOXY AZETIDINE
1-BENZHYDRYLAZETIDIN-3-YL METHANESULFONATE
1-DIPHENYLMETHYL-3-AZETIDINYL METHANESULFONATE
1-(DIPHENYLMETHYL)-3-(METHANESULFONYLOXY)AZETIDINE
BUTTPARK 37\04-14
1-(diphenylmethyl)-3-(methanesulfonyloxy)azetinide
1-Diphenylmethyl-3-azetidinyl Methanethiosulfonate
1-Benzhydryl-3-methanesulphonyloxyazetidine
1-BENZHYDRYL-3-METHANESULFONATOAZETIDINE 98%
Methanesulfonic acid 1-benzhydryl-azetidin-3-yl ester
1-(Diphenylmethyl)-3-(mesyloxy)azetidine
1-(Diphenylmethyl)-3-[(methylsulfonyl)oxy]azetidine | [EINECS(EC#)]
608-857-8 | [Molecular Formula]
C17H19NO3S | [MDL Number]
MFCD00159216 | [Molecular Weight]
317.4 | [MOL File]
33301-41-6.mol |
| Chemical Properties | Back Directory | [Appearance]
White Crystalline Solid | [Melting point ]
111-112°C | [Boiling point ]
459.9±34.0 °C(Predicted) | [density ]
1.28±0.1 g/cm3(Predicted) | [storage temp. ]
Sealed in dry,Room Temperature | [solubility ]
Dichloromethane, Ether, Ethyl Acetate, Methanol | [form ]
Solid | [pka]
5.67±0.10(Predicted) | [color ]
White | [Usage]
A proline analog and proline formation inhibitor | [InChI]
InChI=1S/C17H19NO3S/c1-22(19,20)21-16-12-18(13-16)17(14-8-4-2-5-9-14)15-10-6-3-7-11-15/h2-11,16-17H,12-13H2,1H3 | [InChIKey]
MSVZMUILYMLJCF-UHFFFAOYSA-N | [SMILES]
N1(C(C2=CC=CC=C2)C2=CC=CC=C2)CC(OS(C)(=O)=O)C1 | [CAS DataBase Reference]
33301-41-6(CAS DataBase Reference) |
| Hazard Information | Back Directory | [Chemical Properties]
White Crystalline Solid | [Uses]
A proline analog and proline formation inhibitor | [Synthesis]
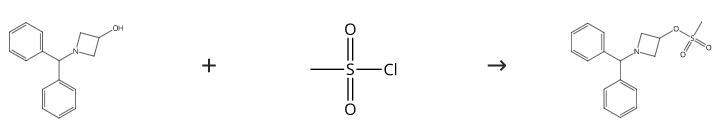
Preparation procedure of 1-(Diphenylmethyl)-3-azetidinyl methanesulfonate: To a reaction flask was charged 632 g (2.64 mol) of 1-benzhydrylazetidin- 3-ol, acetonitrile (1.9 L), and triethylamine (601 g, 1.5 equiv). The mixture was cooled in an ice-acetone bath (-5 °C). Methanesulfonyl chloride (436 g, 1.20 equiv) was added via a drop funnel while keeping the reaction temperature at less than5 °C. HPLC showed reaction completion after 15 min. Water (6.3 L) was added, and the reaction mixture was stirred for 2 h at room temperature and filtered. The filter cake was rinsed with water (2 x 1 L), pulled dry under vacuum, and directly subjected to the amination reaction in the next step. Yield 100%.
 |
|
|